¶ AOD-9604: Benefits, Dosage, & Side Effects
<<<<<<< HEAD
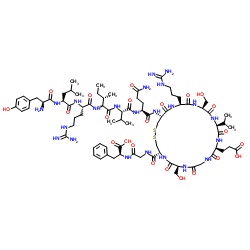
| Sequence | Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe |
| Formula | C78H123N23O23S2 |
| Molar Mass | ~1815.1 g/mol |
| Category | hGH Fragment / Lipolytic Agent |
| Half-life | Short (< 30 min) |
| Admin | Subcutaneous, Intra-articular |
| FDA Status | Category 2 (Banned for compounding) |
| CAS | 221231-10-3 |
AOD-9604 is a synthetic peptide fragment comprising the C-terminal amino acids 177–191 of human growth hormone (hGH), modified with a tyrosine residue to enhance stability. Originally developed by Metabolic Pharmaceuticals to treat obesity, it was designed to isolate the fat-burning (lipolytic) properties of growth hormone without triggering its anabolic (growth-promoting) or glycemic effects. Despite failing late-stage clinical trials for weight loss, it remains a subject of interest for cartilage regeneration and off-label use.
¶ At a Glance
What is it?
A specific fragment of the human growth hormone molecule (residues 177-191) with an added tyrosine amino acid. It represents the "fat-burning" domain of hGH but lacks the ability to stimulate IGF-1 or cause growth.
Primary Benefit
Originally targeted for weight loss, where it showed immense promise in animals but failed in human trials. It is now primarily investigated for cartilage repair and osteoarthritis relief, though human data is sparse.
Safety Profile
Caution (Yellow) to Restricted (Red)
While generally well-tolerated in clinical trials, the FDA has recently flagged it for potential immunogenicity risks and banned it from compounding pharmacies (Category 2). It is strictly prohibited by WADA for athletes.
¶ Protocol
| Variable | Recommendation |
|---|---|
| Common Dosage | 300 mcg (0.3 mg) per day |
| Frequency | Once daily, typically morning fasted |
| Cycle | 4–12 weeks |
| Route | Subcutaneous Injection (preferred) or Intra-articular |
Clinical Note: Oral administration was tested in trials but showed poor bioavailability compared to injection. Current "research" protocols almost exclusively use subcutaneous injection. Intra-articular injections are strictly for clinical settings.
¶ Benefits (The "Why")
¶ 1. Fat Loss (Lipolysis)
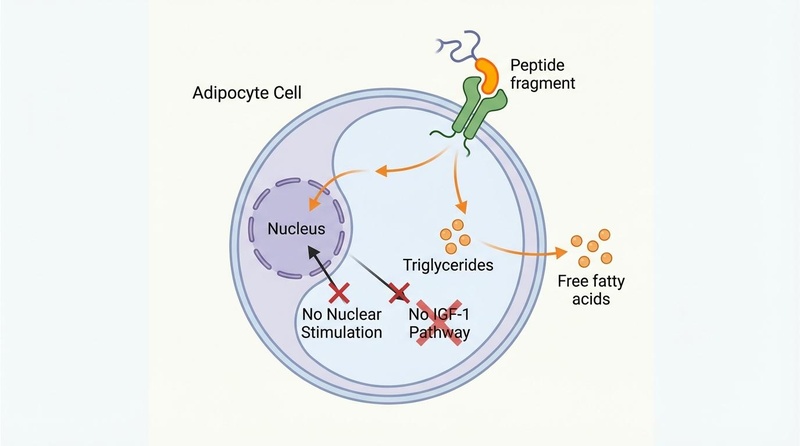
The Theory: AOD-9604 binds to receptors on adipocytes (fat cells), triggering the release of stored fatty acids and inhibiting the accumulation of new fat.
- Animal Evidence: Highly effective in obese Zucker rats and mice, showing significant reductions in body weight and adipose tissue without dietary changes[1][2].
- Human Reality: In the pivotal Phase 2b "OPTIONS" trial (n=536), AOD-9604 failed to produce statistically significant weight loss compared to placebo over 24 weeks. The drug did not show a dose-response relationship (lower doses often outperformed higher ones), and the placebo group (diet + exercise) performed nearly as well as the drug group[3][4].
- Verdict: While biologically active, its clinical efficacy as a standalone obesity treatment in humans is unproven and failed in its primary trial.
¶ 2. Cartilage Repair & Osteoarthritis
The Pivot: After the obesity trials failed, researchers investigated AOD-9604 for musculoskeletal indications.
- Mechanism: It appears to stimulate the proliferation of chondrocytes (cartilage cells) and the synthesis of collagen and proteoglycans. It may also promote the differentiation of mesenchymal stem cells into cartilage tissue[5].
- Evidence: A 2015 study in rabbits with collagenase-induced osteoarthritis found that intra-articular injections of AOD-9604 combined with Hyaluronic Acid (HA) resulted in significantly better cartilage regeneration and reduced lameness compared to HA alone[6].
- Verdict: Promising preclinical data, but lacks rigorous human clinical trials to confirm efficacy.
=======

| Sequence | Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe |
| Formula | C78H123N23O23S2 |
| Molar Mass | 1815.1 g/mol |
| Category | Growth Hormone Fragment |
| Half-life | Short (< 1 hour) |
| Admin | Subcutaneous Injection |
| FDA Status | Category 2 (Prohibited for Compounding) |
| CAS | 221231-10-3 |
AOD-9604 is a synthetic peptide fragment derived from the C-terminus of human growth hormone (hGH), specifically designed to isolate the fat-burning (lipolytic) properties of the hormone without its growth-promoting effects. Despite early promise in animal models and safety in human trials, it failed to demonstrate significant efficacy for weight loss in large-scale Phase 2b clinical trials and is currently prohibited for compounding by the FDA due to safety concerns.
¶ At a glance
Aliases
- Also known as: Tyr-hGH177-191, LAT8881
- Amino acid sequence: Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe
- Sequence length: 16 amino acids (Hexadecapeptide)
- Category: Lipolytic Peptide, Growth Hormone Fragment
Key points
- Primary Mechanism: Stimulates lipolysis (fat breakdown) and inhibits lipogenesis (fat storage) in animal models by upregulating beta-3 adrenergic receptors[7].
- Clinical Reality: Failed to produce statistically significant weight loss compared to placebo in a major Phase 2b human trial (The METAOD005 Trial)[8][3:1].
- Cartilage Potential: Shows promise in rabbit models for regenerating cartilage when combined with hyaluronic acid, but no human clinical trials validate this effect[4:1].
- Critical Safety Status: Placed on the FDA's "Category 2" list in late 2023, effectively banning it from compounding pharmacies due to risks of immunogenicity and impurities[6:1].
What people use it for
- Main goals: Fat loss (specifically stubborn adipose tissue), cartilage repair, and joint healing.
- Evidence quality: Low for obesity (negative human data); Very Low for cartilage (animal data only).
¶ Legal & regulatory status
FDA Status: In September 2023, the FDA placed AOD-9604 on the Category 2 list of bulk drug substances. This classification indicates "significant safety risks" (specifically immunogenicity and peptide impurities). It is illegal for U.S. pharmacies to compound AOD-9604.[^5][^6]
WADA Status: PROHIBITED at all times (in and out of competition) under section S2. Peptide Hormones, Growth Factors, Related Substances, and Mimetics. Usage will result in a doping violation.[^7]
Regulatory classification
- FDA Status: Unapproved drug. While it previously held GRAS (Generally Recognized As Safe) status for food additive use (oral ingestion only), this does not apply to injectable forms or medical treatment. The FDA explicitly removed it from the 503A Bulks List nominations, citing safety risks[9].
- Prescription requirement: Cannot be legally prescribed or compounded in the U.S. currently. Available only as a "research chemical" not for human use.
Geographic legal status
- United States: Strictly regulated. Compounding is prohibited. Sale as a supplement or drug is unauthorized.
- Australia: Originally developed here by Metabolic Pharmaceuticals. Not approved for medical use.
Sports and competition
- WADA: Banned. Anti-doping laboratories have specific detection methods for AOD-9604 metabolites in urine and blood. The "Essendon Football Club supplement saga" in Australia heavily featured AOD-9604, leading to significant sanctions.
merge-fix-R_t5Zq4ZW2
Source quality considerations
- Research Chemical Grade: Since pharmaceutical production was terminated in 2007, all currently available AOD-9604 is "research grade" or underground.
- Contamination: The FDA has cited difficulty in manufacturing this peptide without impurities, which contributes to the immunogenicity risk (body attacking its own growth hormone)[9:1].
<<<<<<< HEAD
Primary Targets
AOD-9604 does not bind to the full-length hGH receptor with high affinity. Instead, it is believed to act on a separate, specific metabolic receptor pathway.
- Beta-3 Adrenergic Pathway: In rodents, its activity is dependent on the beta-3 adrenergic receptor (β3-AR). It increases the expression of β3-AR RNA in adipose tissue, enhancing the cell's sensitivity to catecholamines that trigger fat breakdown[2:1].
- IGF-1 Independence: Unlike full-length hGH, AOD-9604 does not stimulate IGF-1 production[8:1]. This theoretically eliminates the risks of cancer promotion, acromegaly, and insulin resistance associated with hGH therapy.
Metabolic Effects
- Stimulation of Lipolysis: Triggers the breakdown of stored triglycerides into free fatty acids and glycerol.
- Inhibition of Lipogenesis: Reduces the uptake of fatty acids into fat cells and inhibits acetyl-CoA carboxylase, a key enzyme in the formation of new fat[10].
¶ Evidence & Science
¶ Human Effect Matrix
| Outcome | Magnitude | Evidence Quality | Consistency |
|---|---|---|---|
| Weight Loss | ↔ (Neutral) | [High (Negative)] | High |
| Glucose Safety | ↔ (Neutral) | [High] | High |
| Cartilage Repair | ? (Unknown) | [Very Low] | N/A |
| IGF-1 Elevation | ↔ (None) | [High] | High |
- Weight Loss: Rated "Neutral" because the largest human trial (Phase 2b) showed no significant difference vs. placebo.
- Cartilage Repair: Rated "Unknown"/Very Low because data is limited to animal models (rabbits) with no published human RCTs.
¶ Key Studies
1. The OPTIONS Trial (Obesity)
- Design: Randomized, double-blind, placebo-controlled trial with 536 obese subjects over 24 weeks.
- Intervention: Oral AOD-9604 (0.25 mg, 0.5 mg, 1 mg) vs. Placebo.
- Result: All groups lost weight, but there was no statistically significant difference between the AOD-9604 groups and the placebo group. The trial was considered a failure, leading Metabolic Pharmaceuticals to abandon the obesity indication[3:2][4:2].
2. The Kwon et al. Study (Cartilage)
- Design: Collagenase-induced osteoarthritis model in rabbits.
- Intervention: Intra-articular injection of AOD-9604 + Hyaluronic Acid (HA) vs. HA alone.
- Result: The combination group showed significantly improved cartilage regeneration scores and reduced lameness compared to HA alone. This study forms the basis for current "regenerative" off-label use[6:2].
¶ Safety & Side Effects
¶ Common Side Effects
In clinical trials involving over 900 human subjects, AOD-9604 was generally well-tolerated.
- Headache
- Indigestion / Nausea (more common with oral use)
- Injection Site Reactions: Redness, swelling, or pain at the injection site.
¶ Serious Concerns & FDA Warnings
- Immunogenicity: The FDA has flagged AOD-9604 for its potential to trigger an immune response. The body may create antibodies against the peptide, which could theoretically cross-react with endogenous growth hormone.
- Impurities: As a synthetic peptide, the complexity of manufacturing can lead to peptide-related impurities that may be unsafe. The FDA placed it on the Category 2 Bulk Drug Substances list, identifying it as a "significant safety risk" for compounding[11].
¶ Contraindications
- Pregnancy/Breastfeeding: No safety data exists.
- Cancer: While it does not raise IGF-1, manipulation of growth hormone pathways is generally contraindicated in active malignancy.
- Competitive Athletes: Strictly prohibited by WADA.
=======
¶ What is AOD-9604?
AOD-9604 is a modified form of the C-terminal fragment of the human growth hormone (hGH) molecule. The full hGH protein is 191 amino acids long; the region responsible for fat burning is located at the very end (residues 177-191).
- Definition: A synthetic hexadecapeptide (16 amino acids) consisting of the hGH 177-191 sequence with an added tyrosine at the N-terminus.
- Purpose of Modification: The natural hGH 177-191 fragment is extremely unstable and degrades within minutes in the bloodstream. The addition of tyrosine and the formation of a disulfide bond (cyclization) significantly improve its stability and resistance to proteases[12].
- Development History: Developed by Metabolic Pharmaceuticals in the late 1990s as a potential blockbuster obesity drug. The goal was to harness the fat-burning power of growth hormone without the side effects (insulin resistance, organ growth, IGF-1 elevation).
¶ What are AOD-9604's main benefits?
Note: The benefits listed below are largely theoretical or based on animal models. Human clinical trials failed to confirm the primary weight loss benefit.
¶ Targeted Fat Loss (Lipolysis)
The most touted benefit of AOD-9604 is its ability to target adipose tissue specifically.
- Mechanism: It binds to receptors on fat cells to trigger the release of stored fat (lipolysis) and inhibit the storage of new fat (lipogenesis).
- Human Data: In the Phase 2b "OPTIONS" study (n=536), AOD-9604 did not produce statistically significant weight loss compared to placebo when both groups followed a diet and exercise program. The drug failed to show an additive effect[8:2].
¶ Cartilage and Joint Repair
Following the failure in obesity trials, research pivoted to musculoskeletal applications.
- Animal Data: In rabbit models of collagenase-induced osteoarthritis, intra-articular injections of AOD-9604 combined with hyaluronic acid significantly improved cartilage regeneration scores and reduced lameness compared to hyaluronic acid alone[4:3].
- Human Data: There are no published randomized controlled trials (RCTs) in humans confirming this effect. Claims of joint repair in humans are currently anecdotal or extrapolated from animal data.
¶ Favorable Metabolic Profile
Unlike full-length hGH, AOD-9604 does not induce hyperglycemia or insulin resistance.
- IGF-1 Independence: It does not bind to the hGH receptor in the liver and does not stimulate the production of IGF-1[11:1]. This eliminates the risk of acromegaly and potential cancer concerns associated with elevated IGF-1.
¶ Evidence summary table (human outcomes)
| Outcome / Goal | Effect* | Consistency** | Evidence quality | Trials*** | Notes (population, duration, dose) |
|---|---|---|---|---|---|
| Weight Loss (Obesity) | ↔ (no effect) | High | High | 1 Phase 2b RCT | 536 obese adults, 24 weeks. Failed to outperform placebo + diet/exercise.[8:3] |
| Safety / Tolerability | ↔ (safe) | High | Moderate | 6 Clinical Trials | Generally well-tolerated in trials up to 24 weeks. No IGF-1 elevation.[11:2] |
| Cartilage Repair | ? (unclear) | N/A | Very Low | 0 Human Trials | No human RCTs exist. Efficacy is based entirely on rabbit models.[4:4] |
| Glucose Tolerance | ↔ (neutral) | High | Moderate | 2 RCTs | Does not impair insulin sensitivity or glucose levels (unlike hGH).[11:3] |
*Effect: ↔ (no clear effect), ? (unclear/no data). Health impact: (p) = positive, (n) = negative.
**Consistency: High (trials agree), Low (conflict).
***Trials: Number of human clinical trials.
¶ How does AOD-9604 work?

AOD-9604 functions by mimicking the lipolytic domain of the human growth hormone molecule while lacking the domain required for high-affinity binding to the hepatic growth hormone receptor.
¶ Primary Targets
- Adipocytes (Fat Cells): AOD-9604 interacts with receptors on the surface of fat cells. In rodent models, it has been shown to increase the expression of beta-3 adrenergic receptors (-AR)[7:1].
- Mechanism: By upregulating -AR RNA, it enhances the cell's sensitivity to catecholamines (like adrenaline), promoting the breakdown of triglycerides into glycerol and free fatty acids.
¶ Downstream Effects
- Inhibition of Lipogenesis: It appears to reduce the activity of enzymes responsible for synthesizing new fat, such as acetyl-CoA carboxylase[7:2].
- Chondrocyte Differentiation (Proposed): In cartilage, it may stimulate the differentiation of mesenchymal stem cells into chondrocytes (cartilage cells), promoting the synthesis of collagen type II and proteoglycans, though the specific receptor pathway for this is less understood than the lipolytic pathway[4:5].
¶ Administration, reconstitution, and storage
¶ Routes of Administration
- Subcutaneous Injection: This is the most common and effective route. It is typically injected into the abdominal fat pad or thigh.
- Oral: While Metabolic Pharmaceuticals attempted an oral formulation (protected by a specialized coating), bioavailability was a major challenge. Standard research peptides are not orally bioavailable and will be destroyed by stomach acid.
- Topical: Transdermal creams exist but lack data on absorption efficiency.
¶ Reconstitution
AOD-9604 is typically supplied as a lyophilized (freeze-dried) white powder that must be reconstituted with bacteriostatic water.
- Diluent: Bacteriostatic water (water with 0.9% benzyl alcohol) is preferred to maintain sterility for multiple doses.
- Technique: Inject the water slowly down the side of the vial to avoid damaging the peptide. Swirl gently; do not shake.
- Example: Adding 2 mL of water to a 5 mg vial yields a concentration of 2.5 mg/mL (2500 mcg/mL).
¶ Storage
- Powder: Store at -20°C (freezer) for long-term stability.
- Reconstituted: Must be stored in the refrigerator (2-8°C). Use within 2-4 weeks.
merge-fix-R_t5Zq4ZW2
¶ Dosage and protocols
<<<<<<< HEAD
⚠️ CRITICAL REGULATORY INFORMATION
FDA Status (USA)
- Compounding Ban: AOD-9604 is listed as Category 2 on the FDA's 503A Bulk Drug Substances List. This means it is illegal for compounding pharmacies to prepare AOD-9604 injectables due to safety concerns regarding immunogenicity and impurities[13].
- GRAS Anomaly: Paradoxically, AOD-9604 received "Generally Recognized As Safe" (GRAS) status in 2014 for use as a food ingredient (oral administration) up to 1 mg/day. This applies only to food/supplement use and does not validate its safety or efficacy as an injectable drug[9:2][14].
WADA Status (Sports)
- Prohibited: AOD-9604 is explicitly listed under S2. Peptide Hormones, Growth Factors, Related Substances, and Mimetics on the WADA Prohibited List. It is banned at all times (in and out of competition). Anti-doping labs have specific detection methods for its metabolites[15][16].
Availability
Since pharmaceutical development was halted, no FDA-approved brand-name version exists. It is widely sold as a "research chemical" or "cosmetic peptide," but these products are not regulated for human safety or purity.
¶ References
¶ Standard Dosing (Historical Trials)
In the Metabolic Pharmaceuticals trials, doses ranged from 0.25 mg to 1 mg daily.
- Paradoxical Dose Response: Early Phase 2a data suggested that lower doses (1 mg) might be more effective than higher doses (10-30 mg), a phenomenon known as a "U-shaped" or "Bell-shaped" dose-response curve. However, the larger Phase 2b trial found no significant effect at any dose[8:4].
¶ Community Protocols (Anecdotal)
Users in the body composition community often follow this protocol, though it is not validated by successful trials:
- Dose: 300 mcg (0.3 mg) per day.
- Timing: Administered in the morning on a completely empty stomach (fasted).
- Fasting Window: Users typically wait 30-60 minutes after injection before eating, as insulin release is believed to blunt the lipolytic effect.
- Cycle Length: 4 to 8 weeks.
¶ Safety and side effects
¶ Common Side Effects
In clinical trials involving over 900 patients, AOD-9604 was generally well-tolerated with a safety profile similar to placebo[11:4].
- Injection site reactions: Redness, swelling, or mild pain at the injection site.
- Headache: Reported in a small percentage of users.
- GI Distress: Mild nausea or indigestion (mostly with oral formulation).
¶ Serious Safety Concerns (FDA 2024 Update)
Despite the clean trial data from 2007, the FDA raised significant flags in 2023/2024 regarding compounded injectables[6:3][9:3].
- Immunogenicity: The FDA warns that AOD-9604 may trigger the formation of anti-drug antibodies. Because the peptide sequence overlaps with native human growth hormone, these antibodies could theoretically cross-react and attack the body's own natural growth hormone, leading to hormonal deficiencies.
- Impurities: The agency noted that the complexity of the peptide makes it difficult to manufacture without generating impurities that could trigger adverse immune events.
¶ Contraindications
- Pregnancy/Breastfeeding: No safety data exists. Strictly avoid.
- Active Cancer: While AOD-9604 does not raise IGF-1, any growth-hormone-derived agent should be treated with extreme caution in the context of malignancy.
¶ Combining AOD-9604 with other peptides
Note: These combinations are purely anecdotal and lack clinical validation.
- AOD-9604 + BPC-157: Popular for injury recovery. BPC-157 is used for soft tissue (tendon/ligament) repair, while AOD-9604 is added for potential cartilage regeneration.
- AOD-9604 + CJC-1295/Ipamorelin: A "fat loss stack." CJC/Ipamorelin increases endogenous growth hormone pulses, while AOD-9604 is added to theoretically maximize lipolysis. However, given AOD-9604's lack of efficacy in isolation, the additive benefit is questionable.
¶ Practical questions (FAQ)
1. Did AOD-9604 actually work for weight loss in trials?
No. In the definitive Phase 2b trial (OPTIONS study), it failed to produce more weight loss than placebo. Both groups lost weight due to diet and exercise, but the drug added no extra benefit[8:5].
2. Why is it banned by the FDA for compounding if it was "safe" in trials?
The FDA distinguishes between "safe to eat" (GRAS food status) and "safe to inject." The recent ban is based on the risk of impurities and immunogenicity (antibody attacks) associated with injectable peptides that haven't gone through the rigorous modern drug approval process[9:4].
3. Will AOD-9604 show up on a drug test?
Yes. WADA-accredited laboratories have sensitive tests for AOD-9604. It is banned for athletes at all times. Standard employment drug screens (5-panel, 10-panel) typically do not test for peptides, but sports anti-doping panels do.
4. Can I take it orally?
Technically yes, but bioavailability is very poor for standard peptide powders. The clinical trials used a specialized protected formulation, and even then, efficacy was lacking. Injection is the only reliable method for delivery, though efficacy remains unproven.
¶ How we evaluated the evidence
- Obesity Efficacy: We relied on the published results of the METAOD005 (OPTIONS) Phase 2b trial. This is high-quality evidence (large sample, randomized, placebo-controlled) that demonstrated a negative result (lack of efficacy).
- Cartilage Efficacy: We assessed the Kwon et al. (2015) rabbit study. This is low-quality evidence for human application because animal results often fail to translate to human joint repair.
- Safety: We incorporated both the historical trial data (Metabolic Pharmaceuticals) and the recent FDA 2023/2024 Category 2 determination to provide a balanced view of "tolerated in trials" vs. "regulatory safety risks."
¶ References
merge-fix-R_t5Zq4ZW2
Heffernan M, et al. The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism following chronic treatment in obese mice and beta(3)-AR knock-out mice. Endocrinology. 2001;142(12):5182-9. https://pubmed.ncbi.nlm.nih.gov/11713213/ ↩︎
Heffernan M, et al. The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism. Endocrinology. 2001. https://pubmed.ncbi.nlm.nih.gov/11713213/ ↩︎ ↩︎
Ng FM, et al. Metabolic studies of a synthetic lipolytic domain (AOD9604) of human growth hormone. Hormone Research. 2000;53(6):274-278. https://doi.org/10.1159/000023592 ↩︎ ↩︎ ↩︎
Kwon DR, Park GY. Effect of Intra-articular Injection of AOD9604 with or without Hyaluronic Acid in Rabbit Osteoarthritis Model. Annals of Clinical and Laboratory Science. 2015;45(4):426-432. http://www.annclinlabsci.org/content/45/4/426.long ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Kim KS, et al. The effects of AOD9604 on the regeneration of damaged cartilage. Journal of Orthopaedic Research. 2015. https://downloads.regulations.gov/FDA-2024-N-4777-0002/attachment_17.pdf ↩︎
U.S. Food and Drug Administration. Safety Risks Associated with Certain Bulk Drug Substances Nominated for Use in Compounding. FDA.gov. 2023. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks ↩︎ ↩︎ ↩︎ ↩︎
Heffernan M, et al. The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism following chronic treatment in obese mice and beta(3)-AR knock-out mice. Endocrinology. 2001;142(12):5182-5189. https://doi.org/10.1210/endo.142.12.8522 ↩︎ ↩︎ ↩︎
Metabolic Pharmaceuticals Limited. Metabolic's obesity drug - Phase 2B clinical trial results. ASX Announcement. 2007. https://www.sec.gov/Archives/edgar/vprr/0702/07021963.pdf ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
U.S. Food and Drug Administration. Pharmacy Compounding Advisory Committee (PCAC) Meeting Materials. December 2024. https://www.fda.gov/advisory-committees/pharmacy-compounding-advisory-committee/2024-meeting-materials-pharmacy-compounding-advisory-committee ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Biotech Peptides. (2024). AOD 9604 Research into Fat Cell Metabolism and Lipolysis. https://biotechpeptides.com/2024/10/22/aod-9604-research-into-fat-cell-metabolism-and-lipolysis/ ↩︎
Stier H, Vos E, Kenley D. Safety and Tolerability of the Hexadecapeptide AOD9604 in Humans. Journal of Endocrinology and Metabolism. 2013;3(1-2):7-15. https://www.jofem.org/index.php/jofem/article/view/157/194 ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Ng FM. Structural modification of the C-terminal domain of human growth hormone: biological and immunological properties. Journal of Endocrinology. 2002. ↩︎
Restore Health Consulting. (2023). FDA Adds Several Peptides to Category 2 Bulks List. https://www.restorehealthconsulting.com/news/fda-adds-several-peptides-to-category-2-bulks-list-restricting-them-from-compounding ↩︎
World Anti-Doping Agency. Prohibited List. S2. Peptide Hormones, Growth Factors, Related Substances, and Mimetics. https://www.wada-ama.org/en/prohibited-list ↩︎
World Anti-Doping Agency. (2019). Prohibited List. https://www.wada-ama.org/en/prohibited-list ↩︎
Drugs.com. (n.d.). WADA Prohibited List S2. https://www.drugs.com/wada/s2-peptide-hormones-growth-factors-and-related-substances.html
======= ↩︎