¶ ARA-290 (Cibinetide)
¶ At a Glance
¶ What is it?
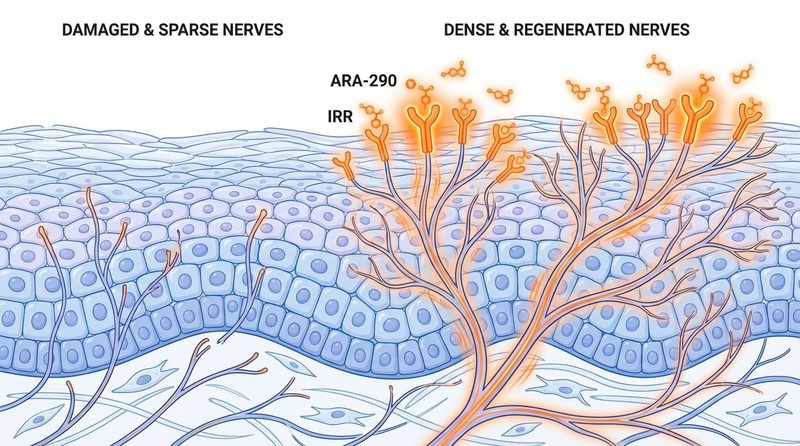
ARA-290 (also known as Cibinetide) is a synthetic 11-amino acid peptide derived from the structure of erythropoietin (EPO). Unlike EPO, which stimulates red blood cell production, ARA-290 is engineered to selectively activate the Innate Repair Receptor (IRR). This receptor triggers potent anti-inflammatory and tissue-repair pathways—specifically promoting the regrowth of small nerve fibers—without thickening the blood or causing cardiovascular risks associated with EPO.
¶ Safety "Traffic Light"
¶ Protocol Card
¶ Standard Protocol (Based on Phase 2b Trials)
- Dosage: 4 mg per day (Subcutaneous injection).
- Frequency: Daily.
- Duration: 28-day cycle (standard clinical trial duration).
- Timing: Any time of day; half-life is short (~20 mins) but effects are sustained.
¶ Bottom Line
ARA-290 is the "Gold Standard" candidate for Small Fiber Neuropathy (SFN), particularly in sarcoidosis and diabetes. It is one of the few interventions clinically proven to regenerate corneal nerve fibers in humans. However, it is currently a "zombie drug"—abandoned by its developer despite successful Phase 2 trials—meaning it is available only as a research chemical.
¶ The "Why": Benefits and Outcomes
¶ 1. Nerve Regeneration (Small Fiber Neuropathy)
The most profound benefit of ARA-290 is its ability to reverse Small Fiber Neuropathy (SFN), a condition characterized by burning pain, autonomic dysfunction, and loss of nerve fibers in the skin and eyes.
- Corneal Nerve Regeneration: In the NERVARA trial, patients taking 4 mg daily showed a significant increase in Corneal Nerve Fiber Density (CNFD)[1].
- Symptom Reduction: Patients reported reduced pain severity and improved quality of life scores[1:1][2].
¶ 2. Anti-Inflammatory & Immunomodulation
ARA-290 does not just block pain signals; it addresses the root cause: inflammation.
- Mechanism: It inhibits the nuclear translocation of NF-B, a master regulator of inflammation, thereby reducing levels of TNF-, IL-6, and IL-1[3][4].
- Sarcoidosis: It has shown specific efficacy in sarcoidosis, a systemic inflammatory disease, by damping down the immune overreaction that damages tissues[1:2].
¶ 3. Metabolic Health (Type 2 Diabetes)
Beyond neuropathy, ARA-290 has shown metabolic benefits in diabetic patients.
- Glycemic Control: In a Phase 2 trial for Type 2 Diabetes, ARA-290 improved HbA1c levels over 28 days[3:1].
- Lipid Profile: It was observed to lower triglycerides and improve cholesterol profiles in the same cohort[3:2].
¶ Reality Check: Context & Availability
¶ The "Zombie Drug" Status
Despite receiving Orphan Drug Designation and Fast Track Status from the FDA for sarcoidosis-associated neuropathy[5][6], commercial development of Cibinetide by Araim Pharmaceuticals appears to have stalled or ceased as of 2024/2025.
- No Active Pharma Source: There is no FDA-approved version available by prescription.
- Research Chemical Only: Patients and biohackers currently source it exclusively from grey-market peptide vendors.
¶ Reconstitution Challenges
A major practical hurdle for users is solubility.
- The "Gel" Issue: When mixed with plain bacteriostatic water, ARA-290 often clumps or forms a gelatinous mass.
- The Fix: Experienced users and compounders use Phosphate Buffered Saline (PBS) or slightly alkaline buffers to ensure it dissolves completely into a clear solution. Injecting a cloudy or gelled solution can be painful and ineffective.
¶ Mechanism of Action
¶ Selective IRR Agonism
Erythropoietin (EPO) has two distinct functions:
- Hematopoietic: Binds to the homodimeric EPO receptor (EPOR-EPOR) to stimulate red blood cell production.
- Tissue Protective: Binds to the Innate Repair Receptor (IRR), a heterocomplex of EPOR and the beta-common receptor (cR or CD131)[7][3:3].
ARA-290 is designed to trigger ONLY #2.
- It is a peptide sequence ("helix B surface peptide") that structurally mimics the region of EPO that binds to CD131 but cannot bind the classical EPO receptor dimer.
- Result: It activates the tissue-repair pathway (JAK2-STAT3, PI3K/Akt) without activating the red-blood-cell pathway (JAK2-STAT5)[3:4][4:1].
¶ The "Molecular Switch"
The IRR is typically not expressed in healthy tissue. It is upregulated on cell surfaces specifically during stress, injury, or inflammation.
- Targeted Action: Because IRR is only present on "stressed" cells, ARA-290 selectively targets injured tissue (like damaged nerves) while bypassing healthy tissue.
- Downstream Effect: Activation suppresses pro-inflammatory cytokines and blocks apoptosis (cell death), allowing the nerve to heal and regrow[8].
¶ Evidence & Science
¶ 1. The NERVARA Trial (Sarcoidosis)
This Phase 2b randomized, double-blind, placebo-controlled trial is the cornerstone of ARA-290 evidence.
- Subjects: 64 patients with sarcoidosis-associated small fiber neuropathy (SSFN).
- Intervention: Daily self-administration of 1 mg, 4 mg, or 8 mg ARA-290 vs. placebo for 28 days.
- Results:
- Nerve Growth: The 4 mg group showed a significant increase in Corneal Nerve Fiber Density (CNFD) compared to placebo ()[1:3].
- Pain Relief: Significant reduction in pain scores and improved functionality (6-minute walk test)[2:1].
- Dose-Response: 4 mg was superior to 1 mg; 8 mg offered no additional benefit.
¶ 2. Type 2 Diabetes Neuropathy Trial
A proof-of-concept study in patients with Type 2 Diabetes and painful neuropathy.
- Protocol: 4 mg ARA-290 daily for 28 days.
- Outcomes: Significant improvement in neuropathic symptoms (PainDetect score) and metabolic markers (HbA1c, lipids)[3:5].
- Safety: No adverse events related to blood counts or cardiovascular strain.
¶ 3. Diabetic Macular Edema (DME)
A pilot study tested ARA-290 for 12 weeks in DME patients.
- Result: While it did not significantly improve central retinal thickness or visual acuity in this small sample, it confirmed safety over a longer duration (12 weeks) and showed improvements in patient-reported vision-related quality of life[9][10].
¶ Comprehensive Safety Profile
ARA-290 is distinguished by its lack of hematopoietic toxicity, a major limitation of using EPO for tissue repair.
¶ Side Effects
- Mild: Injection site reactions (redness, itching) are the most common adverse event.
- Serious: No drug-related serious adverse events (SAEs) were reported in the major Phase 2 trials[1:4][3:6].
- Immunogenicity: No anti-cibinetide antibodies were detected in participants, even after 12 weeks of daily use[9:1].
¶ Contraindications & Interactions
- Current Cancer: As with all growth-factor-related therapies, caution is advised in active malignancy, although ARA-290 is anti-inflammatory and does not stimulate cell proliferation in the same way as growth hormone.
- Pregnancy: No data available; typically contraindicated.
¶ Pharmacokinetics
- Half-Life: Extremely short in plasma (~20 minutes)[11].
- Mechanism of Sustained Effect: The peptide acts as a "hit-and-run" agonist. It briefly binds the receptor, initiating a signaling cascade (phosphorylation of STAT3) that persists for hours or days inside the cell, driving gene expression changes long after the peptide has cleared[11:1].
¶ Comparisons
¶ ARA-290 vs. EPO (Erythropoietin)
| Feature | ARA-290 (Cibinetide) | EPO (Erythropoietin) |
|---|---|---|
| Primary Target | Innate Repair Receptor (IRR) | EPO Receptor (EPOR) Homodimer |
| Effect | Anti-inflammatory, Neuroprotection | Red Blood Cell Production (Hematopoiesis) |
| Side Effects | Injection site pain | Thick blood, stroke risk, hypertension |
| Clinical Use | Neuropathy, Sarcoidosis | Anemia, Kidney Failure |
¶ ARA-290 vs. BPC-157
- BPC-157: Best for gut health, tendon/ligament repair, and general wound healing. Systemic anti-inflammatory but less specific to nerves.
- ARA-290: Specifically targets neuropathy and small fiber regeneration. It is the superior choice for nerve pain, burning sensations, and autoimmune nerve damage.
¶ References
Culver, D. A., et al. (2017). Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Investigative Ophthalmology & Visual Science. https://www.stopsarcoidosis.org/wp-content/uploads/ARAIM-DOSARA-Press-Release.pdf ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Dahan, A., et al. (2016). Targeting the Innate Repair Receptor to Treat Neuropathy (NERVARA Results). Pain Reports. https://pmc.ncbi.nlm.nih.gov/articles/PMC5741312/ ↩︎ ↩︎
Brines, M., et al. (2014). ARA 290, a Nonerythropoietic Peptide Engineered from Erythropoietin, Improves Metabolic Control and Neuropathic Symptoms in Patients with Type 2 Diabetes. Molecular Medicine. https://pmc.ncbi.nlm.nih.gov/articles/PMC4365069/ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Dahan, A., et al. (2013). The erythropoietin analog ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Expert Opinion on Orphan Drugs. https://www.tandfonline.com/doi/full/10.1517/21678707.2013.719289 ↩︎ ↩︎
BioWorld. (2016). Cibinetide receives two FDA orphan drug designations. https://www.bioworld.com/articles/653209-cibinetide-receives-two-fda-orphan-drug-designations ↩︎
PR Newswire. (2014). Araim Pharmaceuticals Given FDA Fast Track Designation to ARA 290 for the Treatment of Sarcoidosis-Associated Small Fiber Neuropathy. https://www.prnewswire.com/news-releases/araim-pharmaceuticals-given-fda-fast-track-designation-to-ara-290-for-the-treatment-of-sarcoidosis-associated-small-fiber-neuropathy-280635872.html ↩︎
Heij, L., et al. (2010). A pilot study on the effect of ARA290 on pain and pain responses. CCMO Register. https://onderzoekmetmensen.nl/en/node/34461/pdf ↩︎
Togel, F., et al. (2016). Cibinetide (ARA 290) for treatment of small fiber neuropathy in sarcoidosis. Expert Opinion on Investigational Drugs. https://www.researchgate.net/publication/260370280_ARA_290_for_treatment_of_small_fiber_neuropathy_in_sarcoidosis ↩︎
McVicar, C. M., et al. (2020). Safety and Efficacy of Cibinetide (ARA 290) in Diabetic Macular Edema: A Phase 2 Pilot Study. Journal of Clinical Medicine. https://www.mdpi.com/2077-0383/9/7/2225 ↩︎ ↩︎
McVicar, C. M., et al. (2020). Diabetic Macular Edema Pilot Study. PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC7408632/ ↩︎
Swartjes, M., et al. (2011). ARA290, a Peptide Derived from the Tertiary Structure of Erythropoietin, Produces Long-Term Relief of Neuropathic Pain. Anesthesiology. https://pmc.ncbi.nlm.nih.gov/articles/PMC3563705/ ↩︎ ↩︎