¶ CJC-1295: Benefits, Dosage, & Side Effects
| Sequence | Tyr-D-Ala-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Leu-Ser-Arg-Lys(Maleimidopropionic acid)-NH2 |
| Formula | C165H269N49O46 (approx) |
| Molar Mass | ~3647.28 g/mol |
| Category | GHRH Analog |
| Half-life | 5–8 days (DAC) ~30 mins (No DAC) |
| Admin | Subcutaneous Injection |
| FDA Status | Unapproved (Compounding Restricted) |
| CAS | 863288-34-0 |
CJC-1295 is a synthetic peptide analog of growth hormone-releasing hormone (GHRH) designed to stimulate the release of growth hormone (GH) and insulin-like growth factor 1 (IGF-1). It is most notable for its ability to bind to serum albumin, extending its half-life from minutes to several days.
Important Distinction: In the peptide community, "CJC-1295" is often sold in two distinct forms:
- CJC-1295 with DAC (The original clinical compound): Active for 5–8 days per injection, creating a continuous "bleed" of GH.
- CJC-1295 without DAC (Modified GRF 1-29): Active for ~30 minutes, requiring multiple daily injections to mimic natural pulsatile release.
¶ At a glance
Aliases
- CJC-1295 with DAC: DAC:GRF, Drug Affinity Complex GHRH.
- CJC-1295 without DAC: Modified GRF 1-29, Mod GRF 1-29, Tetrasubstituted GRF 1-29.
- Category: Growth Hormone Secretagogue (GHS), GHRH Analog.
Key points
- Sustained vs. Pulsatile: The DAC version provides continuous GH elevation ("bleed"), while the No-DAC version mimics natural pulsatile release [1][2].
- Potent IGF-1 Booster: Clinical trials showed massive increases in IGF-1 levels (up to 3x baseline) with the DAC version [1:1].
- Safety Signal: Clinical development was halted in 2006 after a patient death in a Phase II trial, though the death was ruled unrelated to the drug by the attending physician [3].
- Legal Status: As of late 2024, CJC-1295 was removed from the FDA's "Category 2" (prohibited) list but remains under review by the Pharmacy Compounding Advisory Committee (PCAC), which voted against its inclusion for compounding in December 2024 [4].
What people use it for
- Main goals: Increasing muscle mass, fat loss, faster recovery, and anti-aging (IGF-1 elevation).
- Evidence quality: Moderate for physiological effects (GH/IGF-1 levels); Low for long-term clinical outcomes (safety/efficacy balance).
¶ Legal & regulatory status
⚠️ CRITICAL INFORMATION
Regulatory classification
- FDA Status: Unapproved new drug.
- Compounding Status:
- Previous Status: Listed on FDA's "Category 2" (Bulk Drug Substances Raising Significant Safety Concerns), effectively banning it from compounding.
- September 2024 Update: Removed from Category 2 pending further review.
- December 2024 PCAC Vote: The Pharmacy Compounding Advisory Committee (PCAC) voted to recommend against adding CJC-1295 to the 503A Bulks List, citing safety concerns and lack of clinical need. While not a final rule, this effectively keeps it ineligible for legal compounding by 503A pharmacies [4:1].
- Research Use: Widely available as a "research chemical" not for human consumption.
Sports and competition
- WADA Status: Prohibited (Class S2: Peptide Hormones, Growth Factors, Related Substances, and Mimetics). Use is banned in-competition and out-of-competition [5].
Source quality considerations
- Confusion Risk: Many vendors mislabel "Modified GRF 1-29" as "CJC-1295". Users must verify whether the product contains DAC (Drug Affinity Complex).
- Purity: Research grade peptides often lack sterility and purity testing.
¶ What is CJC-1295?
CJC-1295 is a tetrasubstituted analog of the first 29 amino acids of GHRH (Growth Hormone Releasing Hormone).
¶ The "DAC" Difference
The defining feature of true CJC-1295 is the addition of a Drug Affinity Complex (DAC) at the C-terminus.
- Chemistry: A lysine linker attaches a maleimidopropionic acid group to the peptide.
- Mechanism: This group covalently binds to serum albumin (a blood protein) after injection.
- Result: The albumin-peptide complex is too large to be filtered by the kidneys or degraded by enzymes, extending the half-life to 6–8 days [1:2].
¶ The "No DAC" Version (Mod GRF 1-29)
If the DAC is removed, the remaining peptide is Modified GRF 1-29.
- Half-life: ~30 minutes.
- Use: Requires injection 1–3 times daily to mimic natural GH pulses.
- Naming Confusion: Vendors frequently call this "CJC-1295 No DAC", leading to confusion.
¶ What are CJC-1295's main benefits?
Most human data comes from trials of the DAC version.
1. Profound IGF-1 and GH Elevation
In healthy adults, a single injection of CJC-1295 (DAC) increased mean plasma GH concentrations by 2- to 10-fold for 6 days or more. IGF-1 levels increased by 1.5- to 3-fold and remained elevated for up to 28 days following multiple doses [1:3].
2. Muscle and Body Composition (Theoretical)
While direct muscle hypertrophy studies in humans are limited, the sustained elevation of IGF-1 is a potent anabolic signal. Users typically report improved recovery and body composition, aligning with the known effects of elevated GH/IGF-1.
3. "Pulsatile" Secretion Persists
Interestingly, despite the continuous presence of the drug, the pituitary gland maintains some pulsatile character of GH secretion, preventing complete desensitization (tachyphylaxis) that is common with other continuous agonists [2:1].
¶ Evidence summary table (human outcomes)
| Outcome / Goal | Effect | Consistency | Evidence quality | Trials | Notes |
|---|---|---|---|---|---|
| Increase Plasma GH | High | Moderate | 2 RCTs | CJC-1295 DAC. Sustained elevation for >1 week per dose [1:4]. | |
| Increase IGF-1 | High | Moderate | 2 RCTs | Dose-dependent increase (up to 3x baseline) [1:5]. | |
| Safety/Adverse Events | Low | Low | 1 RCT | "No serious adverse reactions" in healthy adults, but lipodystrophy trial halted after patient death [3:1]. |
¶ How does CJC-1295 work?
Primary Target: Growth Hormone-Releasing Hormone Receptor (GHRH-R) in the anterior pituitary.
¶ Mechanism of Action
- Binding: CJC-1295 binds to GHRH receptors, signaling the pituitary to release Growth Hormone (GH).
- Protection (DAC only): The maleimido group reacts with the free thiol group on Cysteine-34 of serum albumin. This forms a stable covalent bond.
- Circulation: The albumin-bound peptide circulates for days, continuously exposing the pituitary to GHRH stimulation.
¶ Pulsatile vs. Continuous Elevation ("GH Bleed")
CJC-1295 with DAC creates a unique physiological state often called "GH bleed"—a continuous, low-level elevation of growth hormone that never returns to baseline. This differs significantly from natural physiology.
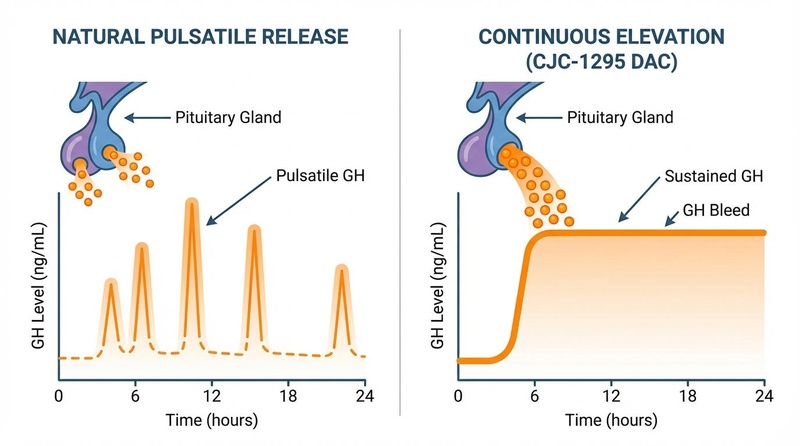
- Natural Release: Sharp, distinct pulses (mostly during sleep), returning to near-zero levels between pulses.
- CJC-1295 (DAC): Elevated basal levels for days. While some pulsatility remains, the trough levels are significantly higher.
- Mod GRF 1-29 (No DAC): Mimics natural release with short duration.
Pharmacokinetics (DAC Version) [1:6]:
- Half-life: 5.8 to 8 days.
- Time to Peak: GH peaks within hours; IGF-1 peaks gradually over days.
- Bioavailability: High (SubQ).
¶ Effects on different systems
¶ Metabolic Health
The sustained elevation of GH and IGF-1 can profoundly impact metabolism. In trials, CJC-1295 increased lipolysis (fat breakdown). However, chronic GH elevation is known to induce insulin resistance. Users of the DAC version often report water retention and potential blood sugar elevations, consistent with acromegaly-like physiology if doses are too high.
¶ Musculoskeletal System
IGF-1 is a primary driver of tissue repair and collagen synthesis. Users report accelerated recovery from injuries and improved joint health. The anabolic effects on muscle are largely mediated through IGF-1, though human data specifically measuring muscle mass gain with CJC-1295 is lacking compared to traditional rhGH therapy.
¶ Skin and Anti-Aging
IGF-1 plays a crucial role in skin elasticity and collagen density. While anecdotal reports often cite "glowing skin" or reduced wrinkles, no formal dermatological studies have been conducted with CJC-1295.
¶ Administration, reconstitution, and storage
Routes of administration
- SubQ (Subcutaneous): The standard route. Injection into belly fat or thigh.
- Frequency:
- DAC Version: Once weekly.
- No DAC Version: 1–3 times daily.
Reconstitution
- Diluent: Bacteriostatic water (essential for multi-dose vials).
- Volume: Typically add 1mL or 2mL to the vial.
- Technique: Inject water slowly down the side of the vial. Swirl gently; do not shake.
Example reconstitution calculations:
| Vial strength | Diluent volume | Final concentration | Dose (100 mcg) | Dose (1000 mcg / 1 mg) |
|---|---|---|---|---|
| 2 mg | 2 mL | 1 mg/mL (1000 mcg/mL) | 0.10 mL (10 units) | 1.0 mL (100 units) |
| 5 mg | 2 mL | 2.5 mg/mL (2500 mcg/mL) | 0.04 mL (4 units) | 0.4 mL (40 units) |
Note: 100 units on a standard insulin syringe = 1 mL.
Storage
- Powder: Freezer (-20°C) for long-term storage (years).
- Reconstituted: Refrigerator (2–8°C).
- Stability:
- DAC Version: Highly stable.
- No DAC Version: Degrades rapidly (weeks) after reconstitution; keep cold and use quickly.
¶ Dosage and protocols
¶ 1. Clinical Trial Protocol (CJC-1295 DAC)
- Dose: 30 to 60 mcg/kg body weight.
- Example: For a 100 kg person, this is 3,000 to 6,000 mcg (3–6 mg) per week.
- Frequency: Weekly or bi-weekly.
- Outcome: Maximum IGF-1 elevation.
¶ 2. Common Community Protocol (CJC-1295 DAC)
- Dose: 1,000 to 2,000 mcg (1–2 mg) per week.
- Frequency: Once weekly (e.g., Monday night).
- Rationale: Lower than trial doses to avoid side effects (water retention) while still boosting basal GH/IGF-1.
¶ 3. Common Community Protocol (Mod GRF 1-29 / "No DAC")
- Dose: 100 mcg per injection.
- Frequency: 1 to 3 times daily (e.g., morning, post-workout, before bed).
- Rationale: Mimics natural pulsatile release; avoids "GH bleed" side effects.
¶ Combining CJC-1295 with Ipamorelin
This is the most popular "stack" in the longevity community.
The Protocol:
- Components: CJC-1295 No DAC (Mod GRF 1-29) + Ipamorelin.
- Mechanism:
- CJC-1295: Amplifies the amount of GH released per pulse (increases signal strength).
- Ipamorelin: Initiates the pulse and inhibits somatostatin (removes the "brake").
- Synergy: Combined, they release significantly more GH than either alone.
- Typical Dose: 100 mcg Mod GRF + 100–200 mcg Ipamorelin, 1–2x daily.
¶ Safety and side effects
Clinical Safety Data
In the Teichman et al. study (healthy adults), adverse events included:
- Injection site reactions: Mild pain, redness, induration (common).
- Systemic: Headache, diarrhea, flushing (warm sensation immediately after injection).
- No Serious Events: In the healthy adult cohort.
The "Lipodystrophy Trial" Death
A Phase II trial for HIV-associated lipodystrophy was halted in 2006 after a patient died.
- Cause: The attending physician stated the death was due to asymptomatic coronary artery disease and unrelated to the drug.
- Consequence: Despite the "unrelated" ruling, the death created a regulatory hurdle that effectively ended commercial development [3:2].
Side Effects of Sustained GH (DAC Version)
Continuous GH elevation ("GH bleed") carries different risks than pulsatile release:
- Water Retention: Significant fluid retention (edema) is common at higher doses.
- Insulin Resistance: Chronic GH elevation can impair insulin sensitivity (diabetogenic effect).
- Carpal Tunnel: Nerve compression due to fluid retention.
- Acromegalic Features: Theoretical risk with long-term, high-dose abuse.
Peptide-specific safety issues
- Antibody Formation: The DAC complex is a foreign structure. While not observed in short trials, long-term use could theoretically trigger immune responses.
- Source Purity: Research grade peptides are not held to pharmaceutical purity standards.
¶ Practical questions (FAQ)
1. Which version is better: DAC or No DAC?
For mimicking natural physiology and minimizing side effects, No DAC (Mod GRF 1-29) is generally preferred. For convenience (one shot per week) and maximum IGF-1 levels (for pure mass), DAC is sometimes used, but carries higher risks of water retention and insulin resistance.
2. How long does it take to see results?
Improved sleep and recovery may be noticed within the first week. Changes in body composition (fat loss, muscle fullness) typically take 8–12 weeks of consistent use.
3. Does CJC-1295 cause cancer?
There is no evidence that CJC-1295 causes cancer, but elevated IGF-1 is a growth factor that can accelerate the growth of existing tumors. Individuals with a history of cancer are typically advised to avoid GH secretagogues.
4. Can I mix CJC-1295 and Ipamorelin in the same syringe?
Yes, they are commonly mixed immediately before injection. Some vendors sell them pre-blended, though this limits dosing flexibility.
5. Is a PCT (Post Cycle Therapy) needed?
Generally, no. Unlike anabolic steroids, CJC-1295 does not shut down natural testosterone production. However, long-term continuous use (DAC) could theoretically desensitize pituitary receptors, so cycling off (e.g., 8 weeks on, 4 weeks off) is recommended.
¶ How we evaluated the evidence
We prioritized human randomized controlled trials (RCTs) for efficacy and safety data.
- Efficacy: Based primarily on the Teichman et al. (2006) and Ionescu et al. (2006) studies, which provided high-quality pharmacokinetic data in healthy humans.
- Safety: Derived from the same clinical trials and the reported adverse events in the halted Phase II trial.
- Community Protocols: Dosing information for "No DAC" protocols is largely derived from widespread community usage and theoretical application of GHRH half-lives, as few specific clinical trials exist for the "No DAC" version in the context of bodybuilding/anti-aging.
¶ References
Teichman, S. L., et al. (2006). Prolonged Stimulation of Growth Hormone (GH) and Insulin-Like Growth Factor I Secretion by CJC-1295, a Long-Acting Analog of GH-Releasing Hormone, in Healthy Adults. Journal of Clinical Endocrinology & Metabolism. https://doi.org/10.1210/jc.2005-1536 ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎
Ionescu, M., & Frohman, L. A. (2006). Pulsatile Secretion of Growth Hormone (GH) and Insulin-Like Growth Factor I (IGF-I) Following CJC-1295 Administration. Journal of Clinical Endocrinology & Metabolism. https://doi.org/10.1210/jc.2006-1702 ↩︎ ↩︎
aidsmap news. (2006). Lipodystrophy study halted after patient death. https://www.aidsmap.com/news/jul-2006/lipodystrophy-study-halted-after-patient-death ↩︎ ↩︎ ↩︎
FDA. (2024). Pharmacy Compounding Advisory Committee Meeting, December 4, 2024. https://www.fda.gov/advisory-committees/advisory-committee-calendar/updated-meeting-time-and-public-participation-information-december-4-2024-meeting-pharmacy ↩︎ ↩︎
WADA. (2024). Prohibited List. https://www.wada-ama.org/en/prohibited-list ↩︎