¶ GHRP-2 (Pralmorelin): Benefits, Dosage, & Side Effects
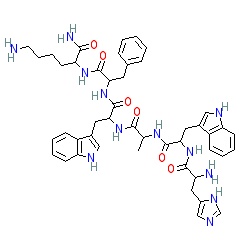
| Sequence | D-Ala-D-2-Nal-Ala-Trp-D-Phe-Lys-NH₂ |
| Formula | C45H55N9O6 |
| Molar Mass | 817.97 g/mol |
| Category | GH Secretagogue (GHS) |
| Half-life | 15–60 minutes |
| Admin | Subcutaneous Injection |
| FDA Status | Category 2 (Restricted) |
| CAS | 158861-67-7 |
GHRP-2 (Pralmorelin) is a potent, second-generation synthetic peptide that stimulates the release of growth hormone (GH). It is widely recognized for being more potent than its predecessor, GHRP-6, while causing significantly less hunger. However, unlike "cleaner" alternatives like Ipamorelin, GHRP-2 has a "dirty" profile: it moderately elevates cortisol and prolactin along with growth hormone[1][2].
¶ At a glance
Aliases
- Generic Name: Pralmorelin (Pralmorelin Hydrochloride)
- Synonyms: KP-102, GPA-748
- Category: Growth Hormone Secretagogue (Ghrelin Agonist)
Key points
- Primary Benefit: Potent stimulation of pulsatile growth hormone release, which can improve body composition and recovery.
- Secondary Effect: significantly increases appetite (approx. 35% increase in food intake), making it useful for bulking but potentially counterproductive for fat loss[3].
- Key Limitation: Short half-life requires frequent dosing (2–3 times daily) for maximum anabolic effect.
- Safety Warning: As of late 2023, the FDA placed GHRP-2 on the Category 2 list, effectively banning it from pharmacy compounding due to safety concerns regarding immunogenicity and insulin resistance[4].
What people use it for
- Main goals: Muscle hypertrophy (bulking), appetite stimulation, injury recovery.
- Evidence quality: High for GH secretion and appetite stimulation; Moderate for long-term body composition changes in healthy adults.
¶ Legal & regulatory status
⚠️ CRITICAL INFORMATION: FDA RESTRICTIONS (2023-2024)
Regulatory classification
- FDA Status: Category 2 (Bulk Drug Substances). In late 2023, the FDA moved GHRP-2 to Category 2, which includes substances presenting "significant safety risks." This designation prohibits 503A compounding pharmacies from creating or selling medications containing GHRP-2[5][6].
- Specific Safety Concerns: The FDA cited risks of immunogenicity (due to peptide impurities and aggregates) and adverse events including increased insulin requirements and fluid retention[4:1].
- Prescription requirement: While technically a prescription drug in some jurisdictions (like Japan, where it is approved as GHRP-2 Kaken for diagnosing GH deficiency), it is currently unavailable through legitimate U.S. compounding pharmacies.
Sports and competition
- WADA status: Prohibited. GHRP-2 is listed under S2 (Peptide Hormones, Growth Factors, Related Substances, and Mimetics). Its use is banned at all times (in- and out-of-competition) for professional athletes.
¶ What is GHRP-2?
Definition
GHRP-2 is a synthetic hexapeptide (chain of 6 amino acids) belonging to the growth hormone-releasing peptide (GHRP) family. It acts as a selective agonist of the ghrelin receptor (GHS-R1a), mimicking the action of ghrelin, the "hunger hormone."
Relationship to other peptides
- Vs. GHRP-6: GHRP-2 is a "second-generation" peptide. It is more potent at releasing GH on a molar basis than GHRP-6 and triggers less extreme hunger, though appetite stimulation is still a major effect[7].
- Vs. Ipamorelin: Ipamorelin is a "third-generation" peptide noted for its selectivity; it releases GH without elevating cortisol or prolactin. GHRP-2, by contrast, stimulates all three, making it less selective but often considered "stronger" for raw mass gain[8].
¶ What are GHRP-2's main benefits?
Growth Hormone Secretion
- Outcome: Significant, dose-dependent elevation of plasma growth hormone.
- Mechanism: GHRP-2 amplifies the natural pulsatile release of GH. Unlike exogenous HGH (which creates a flat, unnatural elevation), GHRP-2 creates sharp spikes in GH levels[1:1].
- Evidence quality: High. Multiple human trials confirm its potency as a diagnostic agent and therapeutic[9][10].
Appetite Stimulation & Weight Gain
- Outcome: Increased voluntary food intake and body weight.
- Magnitude: In a study of healthy men, GHRP-2 infusion increased food intake at a buffet meal by 35.9% compared to placebo[3:1].
- Population: Demonstrated in lean men, obese men, and children with failure to thrive.
- Evidence quality: High. It is a reliable appetite stimulant, often used off-label for "hard gainers" or those needing to overcome caloric deficits.
Linear Growth (in Children)
- Outcome: Increased height velocity.
- Magnitude: Long-term intranasal treatment (twice daily) significantly increased growth rates in GH-deficient children (from 3.7 cm/year to 6.1 cm/year)[9:1].
- Evidence quality: Moderate to High (specific to pediatric GHD populations).
¶ Evidence summary table (human outcomes)
| Outcome / Goal | Effect* | Consistency** | Evidence quality | Trials | Notes |
|---|---|---|---|---|---|
| Peak GH Release | High | High | >10 RCTs | Highly reliable stimulation; used clinically for diagnosis. | |
| Appetite Increase | ↑↑ (n/p) | High | High | 3+ RCTs | ~36% increase in calorie intake; beneficial for bulking, negative for fat loss[3:2]. |
| Cortisol Elevation | High | High | 5+ RCTs | Acute rise in ACTH/Cortisol post-injection; usually within normal range but significant[7:1]. | |
| Prolactin Elevation | High | High | 5+ RCTs | Dose-dependent rise; risk of gynecomastia in sensitive individuals[7:2]. | |
| Lean Body Mass | Moderate | Low | 2 RCTs | Hard data in healthy adults is limited; mostly extrapolated from GH effects. |
*Effect: ↑ (increase), ↓ (decrease). (p) = positive/desirable, (n) = negative/side effect.
**Consistency: High (most trials agree).
¶ How does GHRP-2 work?
Mechanism of Action
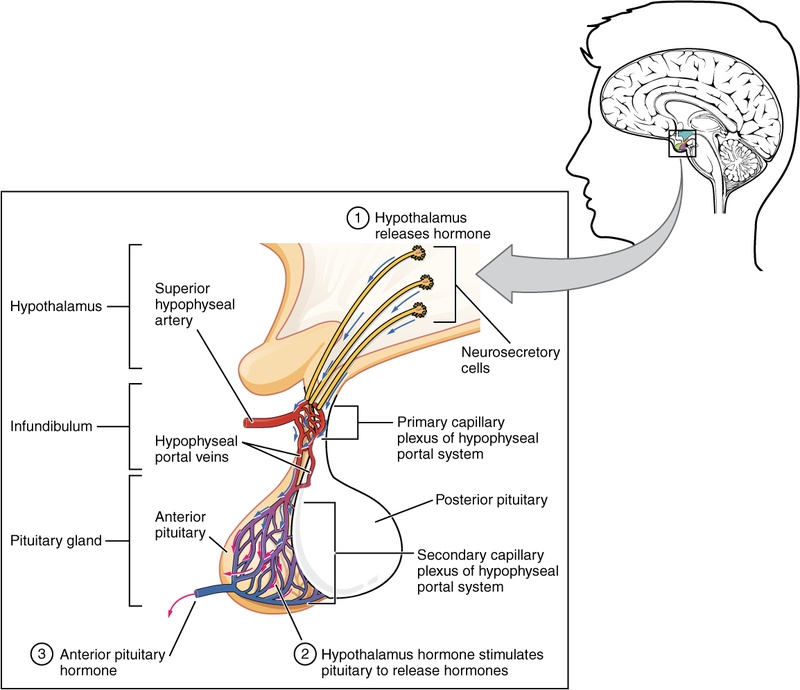
- Receptor Binding: GHRP-2 binds to the Growth Hormone Secretagogue Receptor 1a (GHS-R1a) in the pituitary gland and hypothalamus.
- Signal Transduction: This activation triggers a phospholipid-dependent pathway (IP3/DAG), causing an influx of calcium into pituitary somatotrophs.
- Depolarization & Release: The calcium surge causes these cells to release stored growth hormone vesicles.
- Somatostatin Inhibition: GHRP-2 may also interfere with somatostatin (the hormone that "brakes" GH release), allowing for a larger pulse.
Pulsatile vs. Continuous
Crucially, GHRP-2 maintains the pulsatile nature of GH secretion. The body naturally releases GH in pulses (mostly during sleep). GHRP-2 amplifies these pulses rather than creating a continuous "bleed" of hormone. This is generally safer for insulin sensitivity than continuous exposure, though the "dirty" aspect (cortisol elevation) complicates this safety profile.
Pharmacokinetics
- Half-life: Very short, approximately 15–60 minutes via SubQ injection[10:1].
- Metabolism: Rapidly degraded by blood peptidases.
- Implication: Because it clears so quickly, a single daily dose is ineffective for sustained anabolism. Users typically inject 2–3 times daily to mimic natural GH pulses.
¶ Administration and Reconstitution
Routes of administration
- Subcutaneous (SubQ): The standard and most effective route. Injected into body fat (abdomen) using an insulin syringe.
- Intranasal: Studied in children; effective but requires significantly higher doses (5–20x the injectable dose) to achieve similar serum levels[9:2].
- Oral: Very poor bioavailability. High doses (300x injectable) can stimulate appetite but are inefficient for GH release[11].
Reconstitution
GHRP-2 is sold as a lyophilized (freeze-dried) powder.
- Diluent: Use Bacteriostatic Water (contains 0.9% benzyl alcohol) to prevent bacterial growth.
- Volume: Adding 2mL of water to a 5mg vial creates a concentration of 2.5 mg/mL (2500 mcg/mL).
- Storage: Store powder in the freezer. After reconstitution, keep refrigerated (2–8°C) and use within 4 weeks.
¶ Dosage and protocols
Standard Dosage (Bodybuilding/Off-Label)
- Dose: 100 mcg to 300 mcg per injection.
- Note: The "saturation dose" (where receptor occupancy hits diminishing returns) is often cited as ~1 mcg/kg (approx. 100 mcg). Doses above 200 mcg yield marginally more GH but significantly more cortisol and prolactin[12].
- Frequency: 2 to 3 times daily.
- Example: Morning (fasted), Post-workout, Before bed.
- Timing: Must be taken on an empty stomach (at least 2 hours after a meal, 30 mins before food). Fats and carbohydrates (insulin) blunt the GH release spike.
The "Synergistic Stack"
GHRP-2 is rarely used alone. It is almost always stacked with a GHRH (Growth Hormone Releasing Hormone) analog.
- The Stack: GHRP-2 + CJC-1295 (No DAC) (or Mod GRF 1-29).
- Why: GHRP-2 initiates the pulse; CJC-1295 amplifies the size of that pulse. Used together, they can release significantly more GH than the sum of their individual effects.
¶ Safety and side effects
Common Side Effects
- Hunger: Intense hunger (the "ghrelin munchies") typically hits 20–30 minutes post-injection.
- Water Retention: "Puffy" face or hands due to aldosterone/cortisol activity. Usually subsides after 1–2 weeks.
- Lethargy: A crash or tiredness shortly after dosing (often associated with the GH pulse).
Hormonal Side Effects (The "Dirty" Profile)
Unlike Ipamorelin, GHRP-2 stimulates stress hormones:
- Cortisol: Elevated ACTH leading to cortisol release. This can cause anxiety, sleep disturbance, or catabolism in sensitive users.
- Prolactin: Dose-dependent increase. High prolactin can cause nipple sensitivity or gynecomastia in men.
- Management: Some users take Vitamin B6 (P5P) or Cabergoline to manage prolactin, though switching to Ipamorelin is the safer strategy.
Insulin Resistance
Chronic use of GH secretagogues can elevate blood glucose and reduce insulin sensitivity. The FDA specifically noted "increased insulin requirements" as a safety risk for GHRP-2[4:2].
Contraindications
- Cancer: Active malignancy (GH/IGF-1 promotes cell growth).
- Pregnancy/Breastfeeding: Strictly contraindicated.
- History of Gynecomastia: Due to prolactin risk.
¶ Cost considerations
- Market Status: Since the FDA Category 2 designation (late 2023), legitimate U.S. sources have vanished.
- Gray Market: Research chemical sites sell 5mg vials for $20–$45.
- Value: Moderate. It is cheaper than HGH but requires frequent pinning. For users avoiding hunger or cortisol issues, the slightly more expensive Ipamorelin is often considered better value.
¶ References
Arvat, E., et al. (1997). Growth hormone-releasing activity of hexarelin, GHRP-2, and GHRP-6 in man. European Journal of Endocrinology. https://pubmed.ncbi.nlm.nih.gov/9285939/ ↩︎ ↩︎
Laferrère, B., et al. (2005). Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. Journal of Clinical Endocrinology & Metabolism. https://pubmed.ncbi.nlm.nih.gov/15699539/ ↩︎
Laferrère, B., et al. (2005). Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. Journal of Clinical Endocrinology & Metabolism. https://pubmed.ncbi.nlm.nih.gov/15699539/ ↩︎ ↩︎ ↩︎
FDA. (2023). Category 2 of the Bulk Substances Nominated Under Sections 503A. https://www.fda.gov/media/94155/download ↩︎ ↩︎ ↩︎
FDA. (2023). List of Bulk Drug Substances Nominated for Use in Compounding Under Section 503A. https://www.fda.gov/media/94155/download ↩︎
FDA. (2023/2024). Safety Risks Associated with Certain Bulk Drug Substances for Use in Compounding. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks ↩︎
Arvat, E., et al. (1997). Growth hormone-releasing activity of hexarelin, GHRP-2, and GHRP-6 in man. https://pubmed.ncbi.nlm.nih.gov/9285939/ ↩︎ ↩︎ ↩︎
Peptide Sciences. (2025). Ipamorelin vs GHRP. https://www.peptidesciences.com/peptide-research/ipamorelin-vs-ghrp ↩︎
Pihoker, C., et al. (1997). Treatment effects of intranasal growth hormone releasing peptide-2 in children with short stature. Journal of Endocrinology. https://pubmed.ncbi.nlm.nih.gov/9390009/ ↩︎ ↩︎ ↩︎
Pihoker, C., et al. (1998). Pharmacokinetics and pharmacodynamics of growth hormone-releasing peptide-2: a phase I study in children. https://pubmed.ncbi.nlm.nih.gov/9543135/ ↩︎ ↩︎
Pihoker, C., et al. (2003). Appetite and body weight in response to long-term oral administration of the ghrelin agonist GHRP-2 in growth hormone deficient children. International Journal of Pediatric Endocrinology. https://pubmed.ncbi.nlm.nih.gov/14513874/ ↩︎
Agewell. (2025). Personalized Growth Hormone Peptide Dosing. https://www.agewellatl.net/personalized-growth-hormone-peptide-dosing/ ↩︎