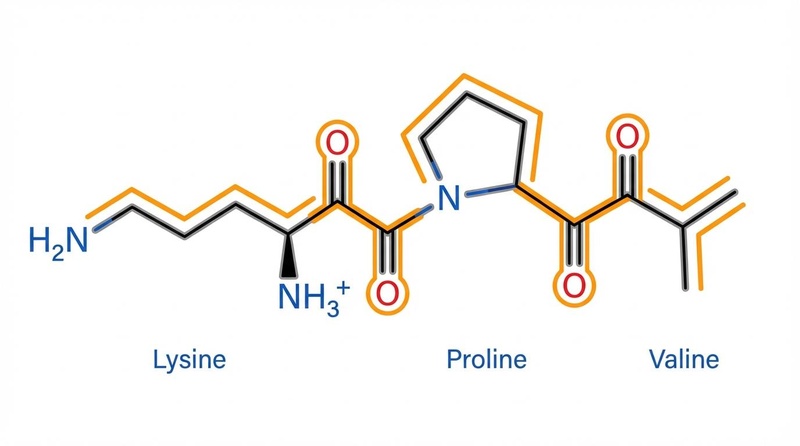
¶ KPV (Lysine-Proline-Valine)
¶ At a Glance
¶ Safety & Regulation
As of September 2023, the FDA placed KPV on the "Category 2" bulk drug substances list, prohibiting its compounding in standard 503A pharmacies due to "insufficient human safety data." It remains a research chemical in the US.
Unlike Melanotan II or full-length $\alpha$-MSH, KPV does not bind to MC1R to induce tanning or darken moles.
Likely prohibited under S2 (Peptide Hormones and Mimetics) as a fragment of ACTH/$\alpha$-MSH. Athletes should strictly avoid.
¶ Protocol Card
Often encapsulated for colonic release or taken as a liquid. Best taken on an empty stomach to utilize PepT1 transport.
Applied 1–2 times daily to affected areas (psoriasis plaques, eczema, acne lesions).
Used for systemic inflammation or when oral administration is ineffective.
Standard peptide cycling to prevent tolerance, though receptor downregulation is less of a concern than with hormonal peptides.
¶ The Bottom Line
KPV is a specialized tool for targeted inflammation control. While BPC-157 is the "general contractor" for tissue repair, KPV is the "firefighter" for inflamed epithelial linings. Its strongest evidence lies in Inflammatory Bowel Disease (UC) and Candidiasis, where its unique transport mechanism (PepT1) allows it to home in on inflamed cells. It is currently one of the few peptides with direct antimicrobial activity against Candida albicans and Staphylococcus aureus.
¶ Benefits & Applications
¶ 1. Gut Health & IBD (Ulcerative Colitis)
KPV's primary claim to fame is its efficacy in models of Inflammatory Bowel Disease (IBD). The transporter PepT1 (SLC15A1) is typically expressed in the small intestine but is significantly upregulated in the colon during inflammation (colitis).
- Targeted Delivery: Because KPV is a substrate for PepT1, it is preferentially absorbed by the inflamed colonic cells that need it most, minimizing systemic waste.
- Cytokine Reduction: Once inside the cell, KPV inhibits the NF-B pathway, reducing the secretion of pro-inflammatory cytokines like TNF- and IL-6.
- Mucosal Healing: Animal studies show KPV accelerates the healing of the mucosal lining and prevents weight loss associated with colitis[1][2].
¶ 2. Antimicrobial & Antifungal Action
KPV is unique among common therapeutic peptides for having direct antimicrobial effects.
- Candida: KPV and its derivatives (like CZEN-002) have shown efficacy in human trials for vulvovaginal candidiasis, killing yeast via membrane permeabilization[3].
- Bacteria: It exhibits bactericidal activity against Staphylococcus aureus (including MRSA) and Cutibacterium acnes, making it a dual-action agent for infected wounds or acne[4].
¶ 3. Skin Conditions (Psoriasis & Eczema)
Topical KPV takes advantage of its small size to penetrate the dermis and downregulate inflammation locally.
- Psoriasis: Case studies in patent literature report rapid reductions in scaling, redness, and itching, comparable to mild corticosteroids but without the skin-thinning side effects[5].
- Wound Healing: By modulating fibroblast activity and reducing inflammation, KPV can accelerate wound closure and potentially reduce scarring (similar to GHK-Cu but via different pathways).
¶ Reality Check: Bioavailability & Sourcing
¶ The Oral Bioavailability Challenge
KPV is a tripeptide (Lys-Pro-Val). While small, it is susceptible to degradation by stomach acid and proteases.
- Protection is Key: Generic oral KPV capsules may have poor bioavailability if not enteric-coated or formulated for delayed release.
- The PepT1 Rescue: However, if the peptide reaches the inflamed tissue (e.g., in the colon), the upregulated PepT1 transporter actively pumps it into cells, potentially salvaging efficacy even with lower stability formulations.
¶ Commercial Context
- Forms: Available as lyophilized powder (injectable), oral capsules (often combined with BPC-157/Larazotide), and topical creams.
- Regulatory Status: The FDA's 2023 move to Category 2 has made legitimate sourcing from US compounding pharmacies difficult. Most current supply comes from research chemical vendors ("Not for Human Consumption").
¶ Deep Dive: Mechanism of Action
¶ The PepT1 "Homing" Mechanism
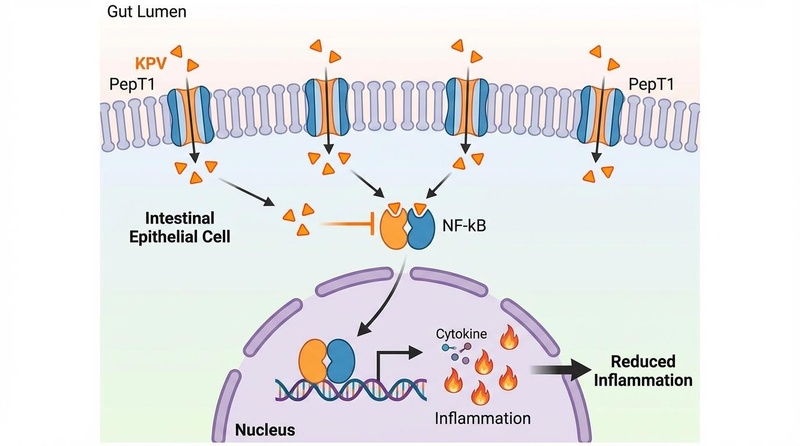
Figure 1: The Targeted Anti-Inflammatory Loop. In healthy colons, PepT1 expression is low. In IBD, epithelial cells upregulate PepT1. KPV utilizes this transporter to enter the cell and block the nuclear translocation of NF-$\kappa$B.
- Transport: KPV binds to the PepT1 transporter (SLC15A1) on the apical membrane of intestinal epithelial cells.
- Intracellular Entry: It is actively transported into the cytoplasm.
- NF-B Inhibition: KPV interacts with Importin-, preventing the p65 subunit of NF-B from entering the nucleus.
- Transcriptional Blockade: Without nuclear access, NF-B cannot trigger the transcription of inflammatory genes (TNF-, IL-1, IL-6).
¶ Antimicrobial Mechanism
KPV's structure mimics the "active site" of -MSH's antimicrobial domain. It disrupts the membrane integrity of fungal cells (Candida) and bacteria (S. aureus), causing leakage of intracellular contents and cell death. This mechanism is distinct from its anti-inflammatory signaling.
¶ Human Effect Matrix
While animal data is robust, human clinical trials are primarily limited to the KPV derivative CZEN-002.
| Condition | Subject/Model | Intervention | Outcome | GRADE | Ref |
|---|---|---|---|---|---|
| Vaginal Candidiasis | Human (n=20) | CZEN-002 Gel (KPV dimer) | 88.2% clinical cure rate; 87.5% mycological cure after 5 days. No adverse events. | High | [3:1] |
| Psoriasis | Human (Case Study) | Topical KPV (1 mg BID) | Marked reduction in erythema, scaling, and pruritus within days; comparable to hydrocortisone. | Very Low | [5:1] |
| Ulcerative Colitis | Animal (Mice) | Oral KPV (in water) | Significant reduction in weight loss and MPO activity; reduced TNF- and IFN-. | Moderate | [1:1] |
| Ulcerative Colitis | Animal (Mice) | HA-KPV Nanoparticles | Superior mucosal healing compared to free KPV due to CD44 targeting and enhanced stability. | Moderate | [2:1] |
| Wound Healing | In Vitro (Caco2) | KPV Solution | Accelerated restitution of wounded epithelial monolayers via cell migration. | Low | [6] |
| Staph Infection | In Vitro | KPV (1 µM) | 95% kill rate of S. aureus (MRSA/MSSA) within 2 hours. | Low | [4:1] |
¶ Safety & Toxicology
¶ Side Effects
- Injection Site: Mild redness or stinging (common with many peptides).
- No Pigmentation: Does not cause tanning, mole darkening, or sexual arousal (unlike Melanotan II).
- Systemic Safety: High doses in animal models showed no organ toxicity or changes in blood chemistry.
¶ Contraindications
- Pregnancy/Breastfeeding: No safety data available. Strictly avoid.
- Active Systemic Infection: While KPV has antimicrobial properties, it is not a substitute for standard antibiotics in life-threatening infections (sepsis, pneumonia).
- Corticosteroid Withdrawal: If using for skin conditions to replace steroids, taper the steroid slowly to avoid rebound effects; do not switch abruptly.
¶ Comparisons
¶ KPV vs. BPC-157
| Feature | KPV | BPC-157 |
|---|---|---|
| Primary Mechanism | NF-B Inhibition, Antimicrobial | Angiogenesis (VEGF), NO Signaling |
| Best For | Active Inflammation (Colitis, Psoriasis), Candida | Tissue Repair (Tendons, Ulcers), Leaky Gut |
| Pigmentation | No | No |
| Synergy | Excellent (The "Firefighter") | Excellent (The "Builder") |
¶ KPV vs. Alpha-MSH / Melanotan
| Feature | KPV | Alpha-MSH / Melanotan II |
|---|---|---|
| Receptor | PepT1 (Transporter) | MC1R, MC3R, MC4R |
| Effect | Anti-inflammatory only | Pigmentation, Arousal, Anti-inflammatory |
| Side Effects | Minimal | Nausea, Flushing, Mole darkening |
¶ References
Dalmasso, G., et al. (2008). PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology, 134(1), 166-178. Link ↩︎ ↩︎
Xiao, B., et al. (2017). Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Molecular Therapy, 25(7), 1628-1640. Link ↩︎ ↩︎
Zengen Inc. (2007). Zengen reports positive phase I/II trial results of its peptide CZEN 002. EurekAlert! Link ↩︎ ↩︎
Cutuli, M. A., et al. (2000). Antimicrobial effects of alpha-MSH peptides. Journal of Leukocyte Biology, 67(2), 233-239. Link ↩︎ ↩︎
Lipton, J. M. (2005). Method for treating dermatitis. US Patent 6,894,028. Link ↩︎ ↩︎
Landy, J., et al. (2012). KPV peptide suppresses NF-kappaB in airway epithelium. Journal of Molecular Medicine. Link ↩︎