¶ P-21 (P021): Benefits, Dosage, & Side Effects
| Sequence | Ac-DGGLAG-NH2 |
| Formula | C30H54N6O5 |
| Molar Mass | ~578.3 g/mol |
| Category | Neurotrophic Peptide |
| Half-life | >3 hours (plasma) |
| Admin | Subcutaneous, Intranasal |
| FDA Status | Research Chemical |
P-21 (also known as P021) is a synthetic peptide mimetic derived from Ciliary Neurotrophic Factor (CNTF). It was developed to bypass the blood-brain barrier (BBB) limitations and side effects of full-length CNTF proteins. Unlike endogenous CNTF, which can cause weight loss and immune reactions, P-21 is designed to robustly stimulate neurogenesis and synaptic plasticity with a favorable safety profile. It is primarily researched for Alzheimer's disease and cognitive enhancement.
¶ At a glance
Aliases
- Names: P021, Peptide 021, P-21.
- Sequence: Ac-Asp-Gly-Gly-Leu-AdamantylGlycine-NH2 (Ac-DGGLAG-NH2).
- Class: CNTF mimetic, neurotrophic factor analog.
Key points
- Neurogenesis: P-21 promotes the growth of new neurons and synapses by mimicking the neurotrophic effects of CNTF and increasing BDNF expression[1].
- Cognitive Enhancement: In animal models, it significantly reverses cognitive deficits associated with Alzheimer's disease and aging[2].
- Safety Advantage: It avoids the anorectic (weight loss) and immunogenic side effects seen with full-length CNTF therapy[3].
- Status: It is currently a research chemical with no completed human clinical trials; all efficacy data is preclinical or anecdotal.
What people use it for
- Goals: Cognitive enhancement ("nootropic"), recovery from Traumatic Brain Injury (TBI), prevention of age-related cognitive decline, and mood stabilization.
- Evidence quality: Low (Strong preclinical data in mice/rats, but zero human clinical trials).
¶ Legal & regulatory status
⚠️ CRITICAL INFORMATION
Regulatory classification
- FDA Status: P-21 is an unapproved research chemical. It has not been evaluated by the FDA for safety or efficacy in humans.
- Compounding: It is generally restricted from use in compounding pharmacies under "Category 2" (Substances with Safety Concerns) or simply due to lack of an USP monograph.
- Availability: It is legally sold by chemical suppliers strictly for "laboratory research use only" and "not for human consumption."
Source quality considerations
- Research Grade: Most available P-21 is synthesized by non-pharmaceutical labs. Purity and identity testing (HPLC/MS) are critical to ensure the presence of the adamantane moiety, which is difficult to synthesize and essential for its stability and BBB penetration.
- Gray Market: Products sold as "nootropics" are unregulated and may contain impurities or incorrect sequences.
¶ What is P-21?
P-21 is a modified tetrapeptide (four amino acids) derived from the active region of human Ciliary Neurotrophic Factor (CNTF), specifically residues 148–151.
The "Adamantane" Innovation
The defining feature of P-21 is the addition of an adamantane group (a tricyclic cage-like hydrocarbon) to the C-terminal glycine. This modification serves three critical functions:
- Lipophilicity: It makes the peptide more fat-soluble, allowing it to cross the Blood-Brain Barrier (BBB) efficiently.
- Stability: It protects the peptide from degradation by peptidases in the blood and gut, extending its half-life to over 3 hours (compared to minutes for unmodified peptides)[4].
- Potency: It stabilizes the bioactive conformation required for receptor interaction.
Relationship to Cerebrolysin
P-21 is often described as a "synthetic Cerebrolysin." Cerebrolysin is a porcine brain extract containing hundreds of peptides, including fragments of CNTF. P-21 was developed by isolating the specific sequence responsible for CNTF's neurogenic properties (originally identified as "Peptide 6") and optimizing it for human use[5].
¶ Main Benefits (Preclinical Evidence)
Note: The following benefits are based on high-quality rodent studies (Tier 2 evidence). Human efficacy has not been confirmed in clinical trials.
¶ 1. Neurogenesis and Synaptic Plasticity
P-21 has been shown to robustly increase the proliferation and differentiation of neural progenitor cells in the dentate gyrus of the hippocampus, a brain region critical for memory formation.
- Mechanism: It increases the expression of Brain-Derived Neurotrophic Factor (BDNF) and phosphorylated CREB (pCREB).
- Outcome: In aged rats, P-21 treatment restored neurogenesis levels to those seen in young adults[6].
¶ 2. Alzheimer's Disease and Neurodegeneration
Extensive research by Dr. Khalid Iqbal's laboratory has utilized P-21 in triple-transgenic Alzheimer's mice (3xTg-AD).
- Tau Pathology: P-21 inhibits GSK-3β (a kinase), thereby reducing the hyperphosphorylation of Tau protein, which forms the "tangles" in Alzheimer's brains[2:1].
- Cognitive Rescue: Treatment prevented and reversed memory deficits in spatial maze tasks and object recognition tests.
- Amyloid Beta: It reduced soluble amyloid-beta levels and plaque pathology, though its effect on Tau is considered more dominant.
¶ 3. Cognitive Enhancement in Normal Aging
In models of healthy aging (Fisher rats), P-21 administration improved discrimination learning and spatial memory, suggesting potential utility for age-related cognitive decline even in the absence of specific pathology[6:1].
¶ Mechanism of Action
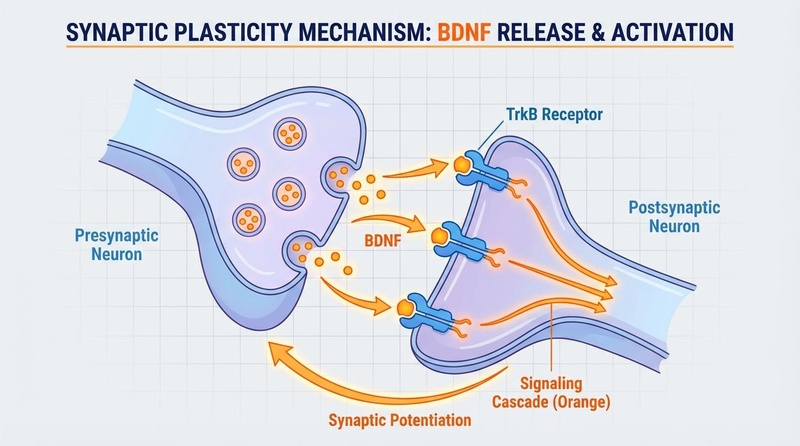
P-21 operates through a unique "disinhibition" mechanism regarding neurogenesis.
Primary Pathway: LIF Signaling Modulation
- The Brake: Under normal adult conditions, a cytokine called Leukemia Inhibitory Factor (LIF) acts as a "brake" on neurogenesis by signaling through the STAT3 pathway.
- The Release: P-21 competitively inhibits LIF signaling. By blocking this inhibitory signal, it effectively "releases the brake," allowing neural stem cells to proliferate[7].
Downstream Signaling
- BDNF Upregulation: The inhibition of LIF signaling leads to a compensatory increase in BDNF expression.
- TrkB Activation: BDNF binds to TrkB receptors, activating the PI3K-Akt pathway.
- GSK-3β Inhibition: Akt phosphorylates and inhibits GSK-3β. Since active GSK-3β promotes Tau phosphorylation and cell death, its inhibition is neuroprotective.
¶ Dosage and Protocols
Warning: These protocols are derived from anecdotal user reports and preclinical extrapolations. They are not medical advice.
¶ Administration Routes
- Subcutaneous (SubQ) Injection: The most common and reliable method. Users typically inject into abdominal fat.
- Intranasal (IN): Available as a spray. While the adamantane group improves mucosal absorption, bioavailability is likely lower than injection.
¶ Common User Protocols
| Goal | Route | Dosage | Frequency | Cycle Length |
|---|---|---|---|---|
| Cognitive Enhancement | SubQ | 100–500 mcg | Once daily | 4–6 weeks |
| Recovery / Repair | SubQ | 500 mcg – 1 mg | Once daily | 4 weeks |
| Intranasal Use | IN | 500 mcg – 2 mg | 1–2x daily | As needed |
Cycling: Unlike acute stimulants, P-21 is believed to require cumulative dosing. Users typically run cycles of 30 days on, followed by 2-4 weeks off to assess baseline function and prevent potential tolerance.
¶ Safety and Side Effects
Preclinical Safety Data
In rodent toxicity studies, P-21 displayed a high safety margin:
- No Weight Loss: Unlike CNTF, it did not cause cachexia (muscle/fat wasting).
- No Immunogenicity: It did not trigger the formation of neutralizing antibodies, a common failure point for protein drugs like Axokine (recombinant CNTF)[3:1].
- Acute Toxicity: Safe in mice at doses up to 550-fold higher than the therapeutic dose.
User-Reported Side Effects
- Fatigue: The most common complaint is immediate drowsiness or a "napogenic" effect shortly after administration. Many users prefer evening dosing for this reason.
- Brain Fog: Paradoxically, some users report temporary brain fog or headache, particularly with intranasal administration.
- Injection Site Reactions: Mild redness or itching (rare).
¶ Comparisons
¶ P-21 vs. Cerebrolysin
- Composition: Cerebrolysin is a complex "soup" of porcine peptides; P-21 is a single synthetic molecule.
- Volume: Cerebrolysin requires large IM injections (5ml+) or IV infusion; P-21 is effective in micro-doses (0.1ml SubQ).
- Safety: P-21 carries zero risk of prion contamination or allergic reaction to porcine proteins, though it lacks Cerebrolysin's decades of human safety data.
¶ P-21 vs. Semax
- Mechanism: Semax modulates ACTH/melanocortin receptors and increases BDNF/NGF; P-21 targets the CNTF/LIF pathway.
- Effect Profile: Semax is often felt as stimulating and acute (focus, clarity); P-21 is described as subtle, cumulative, and reparative (memory, mood stability).
¶ References
Baazaoui, N., & Iqbal, K. (2017). Prevention of Amyloid-β and Tau Pathologies, Associated Neurodegeneration, and Cognitive Deficit by Early Treatment with a Neurotrophic Compound. Journal of Alzheimer's Disease. https://doi.org/10.3233/JAD-170068 ↩︎
Baazaoui, N., et al. (2022). Alzheimer's Disease: Challenges and a Therapeutic Opportunity to Treat It with a Neurotrophic Compound. Biomolecules. https://doi.org/10.3390/biom12101409 ↩︎ ↩︎
Li, B., et al. (2010). Neurotrophic peptides incorporating adamantane improve learning and memory, promote neurogenesis and synaptic plasticity in mice. FEBS Letters. https://doi.org/10.1016/j.febslet.2010.09.035 ↩︎ ↩︎
Ciani, E., et al. (2024). P021 Treatment in CDKL5 Deficiency Disorder. PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10850000/ ↩︎
Iqbal, K., et al. (2014). Method of treating neurofibrillary tangles and/or amyloids beta (Abeta) associated pathologies. US Patent 8796214B2. https://patents.google.com/patent/US8796214B2/en ↩︎
Wei, W., et al. (2019). Neurotrophic Treatment Initiated During Early Postnatal Development Prevents Cognitive Deficits. Frontiers in Aging Neuroscience. https://doi.org/10.3389/fnagi.2019.00224 ↩︎ ↩︎
Chojnacki, A., & Weiss, S. (2004). Leukemia inhibitory factor promotes neural stem cell proliferation in the adult hippocampus. Journal of Neuroscience. https://doi.org/10.1523/JNEUROSCI.5488-03.2004 ↩︎