¶ PT-141 (Bremelanotide): Benefits, Dosage, & Side Effects
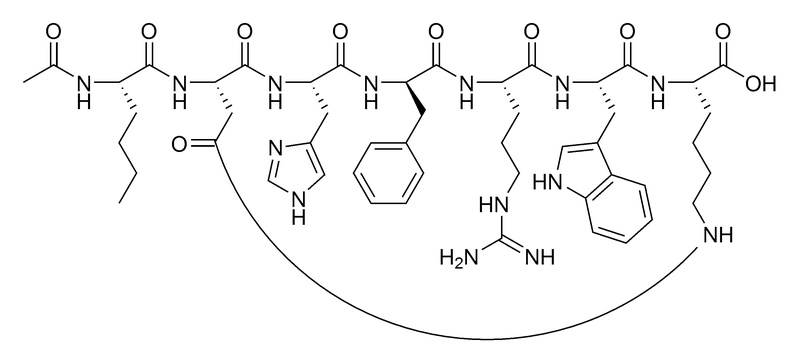
| Sequence | Ac-Nle-Asp-His-D-Phe-Arg-Trp-Lys-OH (Cyclic) |
| Formula | C50H68N14O10 |
| Molar Mass | 1025.18 g/mol |
| Category | Melanocortin Receptor Agonist |
| Half-life | ~2.7 hours (SubQ) |
| Admin | Subcutaneous Injection |
| FDA Status | Approved (Women HSDD) |
| CAS | 189691-06-3 |
| WADA Status | Prohibited (S2 Peptide Hormones) |
PT-141 (Bremelanotide) is a synthetic peptide and FDA-approved therapy (as Vyleesi) that treats sexual dysfunction by acting directly on the central nervous system rather than the vascular system. Unlike traditional ED drugs like sildenafil (Viagra), which facilitate blood flow, PT-141 modulates brain pathways to increase sexual desire and arousal.
¶ At a Glance
Aliases
- Also known as: Bremelanotide, Vyleesi, PT-141 acetate
- Amino acid sequence: Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH
- Sequence length: Cyclic heptapeptide (7 amino acids)
- Category: Melanocortin analog (derivative of Melanotan II)
Key points
- Strongest Benefit: FDA-approved for treating Hypoactive Sexual Desire Disorder (HSDD) in premenopausal women, showing significant improvements in sexual desire and distress scores [1].
- Male Efficacy: Shows efficacy in men with Erectile Dysfunction (ED), including roughly 33% of patients who do not respond to PDE5 inhibitors (sildenafil/tadalafil) [2].
- Key Limitation: High incidence of nausea (up to 40%) which can limit tolerability for some users and requires careful dose titration.
- Safety Concern: Causes transient increases in blood pressure (2–4 mmHg); contraindicated in uncontrolled hypertension and cardiovascular disease.
What people use it for
- Main goals: Restoring sexual desire (libido), treating psychogenic erectile dysfunction, enhancing sexual arousal.
- Evidence quality: High for HSDD (FDA approved); Moderate for male ED (Phase 2 data).
¶ Legal & Regulatory Status
⚠️ CRITICAL INFORMATION
Regulatory classification
- FDA Status: Approved (2019) under the brand name Vyleesi for the treatment of acquired, generalized Hypoactive Sexual Desire Disorder (HSDD) in premenopausal women.
- Prescription Requirement: Prescription required for Vyleesi. Compounded PT-141 is widely available but falls into a regulatory gray area following FDA restrictions on compounding peptides (Category 2 of the Bulk Drug Substances List).
- DEA Schedule: Not a controlled substance.
Geographic legal status
- United States: Vyleesi is FDA-approved. Research chemical PT-141 is sold "for research purposes only."
- International: Approval status varies; widely available as a research chemical globally.
Sports and competition
- WADA Status: Prohibited. While not explicitly named on the 2025 Prohibited List, it falls under Section S2 ("Peptide Hormones, Growth Factors, Related Substances, and Mimetics") as a melanocortin agonist. Its parent compound, Melanotan II, is frequently flagged. Athletes should avoid use.
Source quality considerations
- "Research chemical" PT-141 is widely sold online but lacks the quality assurance of pharmaceutical Vyleesi.
- Purity varies significantly; acetate salts (common in research peptides) mean the actual peptide content is often ~80-85% of the total weight (the rest being counterions and water).
¶ What is PT-141?
PT-141 (Bremelanotide) is a cyclic heptapeptide originally developed from Melanotan II, a tanning peptide. During clinical trials for Melanotan II, researchers observed that it caused spontaneous erections and increased sexual arousal as a side effect. PT-141 was subsequently engineered to retain these aphrodisiac properties while minimizing the melanogenic (skin tanning) effects of its parent compound [3].
It is unique among sexual health interventions because it is a central nervous system (CNS) agent. While PDE5 inhibitors (Viagra, Cialis) work mechanically on the blood vessels of the genitals ("hydraulics"), PT-141 works centrally on the brain's hypothalamus to "switch on" the desire and arousal signaling cascade.
¶ Main Benefits
¶ Sexual Health (Women)
PT-141 is the only injectable medication approved for Hypoactive Sexual Desire Disorder (HSDD).
- Outcome: Increased sexual desire and reduced distress associated with low libido.
- Magnitude: In Phase 3 trials (RECONNECT), women experienced statistically significant improvements in desire scores (FSFI-D) compared to placebo (mean change 0.30–0.42 vs 0.0) [1:1].
- Population: Premenopausal women with acquired, generalized HSDD.
- Evidence Quality: High (FDA Approval based on two large Phase 3 RCTs, n=1,267).
¶ Sexual Health (Men)
Used off-label for Erectile Dysfunction (ED) and low libido.
- Outcome: Improved erectile hardness and duration.
- Key Finding: Effective in men with "psychogenic" ED and those who have failed PDE5 inhibitor therapy. One study showed a 33% response rate in sildenafil non-responders [2:1].
- Evidence Quality: Moderate (Phase 2 trials and extensive clinical usage).
¶ Neuroprotection & Inflammation
Emerging research suggests melanocortin agonists may have broader systemic benefits.
- Potential: Animal models of stroke and renal ischemia suggest MC4R activation reduces inflammation (lowering TNF-α, IL-6) and neuronal damage [4].
- Status: Very Low/Preclinical. This has not been validated in human clinical trials for these indications, though trials for diabetic kidney disease are exploring these anti-inflammatory pathways.
¶ Evidence Summary Table
| Outcome | Effect | Consistency | Evidence Quality | Trials | Notes |
|---|---|---|---|---|---|
| HSDD (Women) | High | High | 2 RCTs | FDA-approved indication (RECONNECT studies, n=1,267). Significant improvement in desire and distress. | |
| Erectile Dysfunction | Moderate | Moderate | 3+ RCTs | Phase 2 data showing efficacy in men, including 33% of sildenafil non-responders. | |
| BP Elevation | High | High | All | Transient rise in systolic BP (2-4 mmHg) lasting ~12 hours. | |
| Nausea | High | High | All | Very common side effect (40%), dose-dependent. |
¶ Mechanism of Action
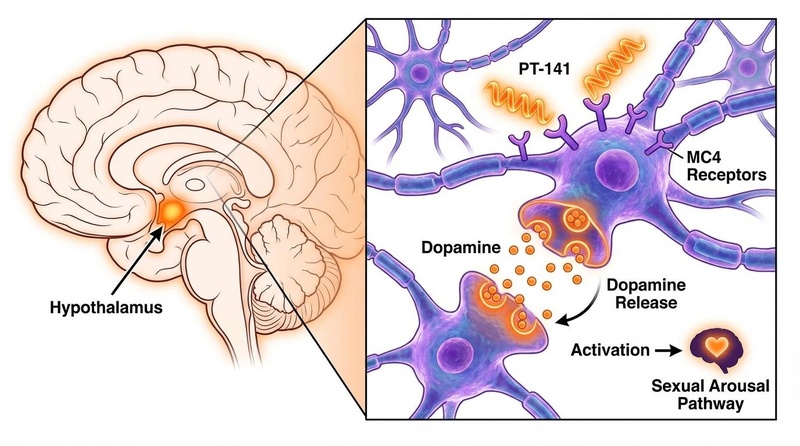
PT-141 acts as a non-selective agonist of melanocortin receptors, with high affinity for MC3R and MC4R.
- Central Activation: The peptide crosses the blood-brain barrier and binds to MC4R receptors in the medial preoptic area (mPOA) of the hypothalamus, a brain region critical for sexual behavior.
- Dopamine Release: Activation of these presynaptic receptors triggers the release of dopamine, a neurotransmitter associated with reward, motivation, and arousal. It may also modulate oxytocin levels [5].
- Sympathetic Modulation: This central signal modulates the sympathetic nervous system to initiate the physiological response of sexual arousal (lubrication in women, erection in men) independent of direct physical stimulation.
Contrast with PDE5 Inhibitors:
- PDE5i (Viagra/Cialis): Peripheral mechanism. Inhibits the PDE5 enzyme in blood vessels, preventing blood from leaving the penis. Requires sexual stimulation to work.
- PT-141: Central mechanism. Acts on the brain to create the arousal signal itself. Can induce response without stimulation ("spontaneous" arousal).
¶ Effects on Different Systems
¶ Nervous System & Sexual Function
The primary action is in the CNS. By stimulating MC4 receptors, PT-141 directly amplifies the neural "drive" for sex. This makes it particularly effective for psychogenic ED or low libido, where the machinery (blood vessels) works, but the signal (desire) is absent.
¶ Cardiovascular System
PT-141 has a known pressor effect. It causes a transient increase in systolic blood pressure (typically 2–4 mmHg) and a slight decrease in heart rate.
- Timing: Peaks 2–4 hours post-dose and resolves within 12 hours.
- Risk: While negligible for healthy individuals, this can be dangerous for those with uncontrolled hypertension.
¶ Skin (Melanogenesis)
Although designed to minimize tanning compared to Melanotan II, PT-141 still retains some affinity for MC1R (the receptor on melanocytes).
- Result: Frequent use (daily) can lead to focal hyperpigmentation—darkening of the face, gums, or breasts. This occurred in ~1% of clinical trial participants and may not be reversible [1:2].
¶ Administration & Protocols
¶ Routes of Administration
- Subcutaneous Injection (Standard): The only FDA-approved route. Bioavailability is ~100%. Injection is typically done in the abdomen or thigh.
- Intranasal (Research): Investigated but abandoned for pharmaceutical development due to variable absorption and higher rates of adverse blood pressure events. Requires significantly higher doses (often 2-3x the injectable dose) to achieve similar effects.
- Oral: Not effective due to enzymatic degradation in the stomach.
¶ Reconstitution (Research Peptides)
Most research PT-141 comes as a lyophilized (freeze-dried) powder (e.g., 10 mg vial).
- Diluent: Bacteriostatic water (Bac water).
- Technique: Inject diluent slowly down the side of the vial. Swirl gently; do not shake.
- Storage: Store powder in freezer (-20°C). Reconstituted solution must be refrigerated (2-8°C) and used within ~30 days.
Example Reconstitution Calculations:
| Vial Strength | Diluent Volume | Final Concentration | 1.0 mg Dose | 1.75 mg Dose |
|---|---|---|---|---|
| 10 mg | 2 mL | 5 mg/mL | 0.20 mL (20 units) | 0.35 mL (35 units) |
| 10 mg | 5 mL | 2 mg/mL | 0.50 mL (50 units) | 0.875 mL (87.5 units) |
Note: 100 units on a standard insulin syringe = 1 mL.
¶ Dosage and Protocols
Standard FDA Dose (Women - HSDD):
- 1.75 mg via subcutaneous injection.
- Administer at least 45 minutes before anticipated sexual activity.
Off-Label Protocols (Men - ED):
- Starting Dose: 1 mg subcutaneous to assess tolerance (specifically nausea).
- Common Range: 1.5 mg – 2 mg.
- Titration: Increase by 0.25 mg if needed, up to a max of 2 mg.
- Warning: Doses above 2 mg significantly increase the risk of severe nausea and hypertension without providing additional benefit.
¶ Cycling & Timing
- Frequency Limit: Do not exceed one dose every 24 hours.
- Monthly Limit: Do not exceed 8 doses per month. This is critical to prevent receptor desensitization and persistent skin hyperpigmentation.
- Timing: Onset is typically 30–60 minutes, but the "window of opportunity" (increased receptivity) can last 8–12 hours.
¶ Safety & Side Effects
¶ Common Side Effects
- Nausea (40%): The most prevalent complaint. Often mild-to-moderate but can be severe.
- Tip: Some users find that taking an antihistamine or ginger supplement beforehand helps, though clinical evidence for this is anecdotal. It often subsides with repeated use.
- Flushing (20%): Redness and warmth in the face and chest.
- Headache (11%): Typically mild.
- Injection Site Reactions: Pain, itching, or bruising at the injection site (~13%).
¶ Serious Risks & Contraindications
- Blood Pressure: Contraindicated in patients with uncontrolled hypertension or known cardiovascular disease.
- Focal Hyperpigmentation: Darkening of the skin (gums, face, breasts). May be permanent.
- Drug Interactions:
- Naltrexone: PT-141 slows gastric emptying and significantly reduces the absorption of oral naltrexone. Avoid concurrent use [6].
- Alcohol: May increase the risk of flushing and nausea.
¶ Comparison: PT-141 vs. PDE5 Inhibitors
| Feature | PT-141 (Bremelanotide) | Sildenafil / Tadalafil (PDE5i) |
|---|---|---|
| Primary Target | Brain (MC4 Receptor) | Blood Vessels (PDE5 Enzyme) |
| Driver | Increases Desire (Libido) | Facilitates Function (Erection) |
| Requirement | Can work without stimulation | Requires sexual stimulation |
| Onset | 45 minutes | 30–60 minutes |
| Duration | 8–12 hours | 4 hours (Sildenafil) / 36 hours (Tadalafil) |
| Side Effects | Nausea, BP elevation, Flushing | Headache, Stuffy nose, Back pain, Vision changes |
| Best For | Low libido, Psychogenic ED, PDE5 Non-responders | Physical ED, Performance anxiety |
¶ Cost Considerations
- Pharmaceutical (Vyleesi): Can be expensive (~$500-$800 for a pack of 4 autoinjectors) but often covered by insurance for diagnosed HSDD.
- Research Chemical: significantly cheaper (~$30-$50 for a 10mg vial), but lacks purity guarantees and is not for human use.
- Value: For non-responders to cheap generic sildenafil, PT-141 represents a high-value "salvage" therapy, albeit with a higher side-effect burden.
¶ FAQ
Q: Can men take PT-141?
A: While FDA-approved only for women, it is widely prescribed off-label for men. Clinical trials in men showed efficacy for erectile dysfunction, but it was not pursued for approval in this population commercially.
Q: Does it act like Viagra?
A: No. Viagra makes it easier to get an erection if you are aroused. PT-141 creates the arousal itself. Many users describe it as a "mental switch" for desire.
Q: Will it make me tan?
A: It was designed to minimize tanning compared to Melanotan II, but it still has some affinity for skin receptors. Occasional use (less than 8 times a month) generally does not cause noticeable tanning, but focal hyperpigmentation is a known risk.
Q: Why does it cause nausea?
A: The MC4 receptors it targets are also located in the vagus nerve and gastrointestinal tract, which can trigger the nausea response.
Q: How long does it take to work?
A: Most users feel effects within 30-60 minutes, but individual response varies. It is recommended to take it at least 45 minutes before activity.
¶ How we evaluated the evidence
- Study types prioritized: We relied primarily on the FDA approval data packages (RECONNECT Phase 3 trials) for safety and efficacy in women. For male applications, we utilized Phase 2 randomized controlled trials.
- Evidence Quality:
- High: HSDD data (large, multi-center RCTs).
- Moderate: Male ED data (smaller RCTs, consistent positive signal).
- Very Low: Neuroprotection claims (animal models only).
- Distinction: We clearly distinguish between the FDA-approved product (Vyleesi) and research chemicals regarding quality and regulatory status.
¶ References
Kingsberg SA, et al. Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: Two Randomized Phase 3 Trials. Obstet Gynecol. 2019;134(5):899-908. https://doi.org/10.1097/AOG.0000000000003500 ↩︎ ↩︎ ↩︎
Safarinejad MR. Salvage of sildenafil failures with bremelanotide: a randomized, double-blind, placebo controlled study. J Urol. 2008;179(3):1066-1071. https://doi.org/10.1016/j.juro.2007.10.063 ↩︎ ↩︎
Molinoff PB, et al. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann N Y Acad Sci. 2003;994:96-102. https://doi.org/10.1111/j.1749-6632.2003.tb03167.x ↩︎
Giuliani D, et al. Melanocortins as potential therapeutic agents in severe acute brain damage. CNS Neurol Disord Drug Targets. 2011;10(6):666-677. https://doi.org/10.2174/187152711797247849 ↩︎
Pfaus JG, et al. The neurobiology of bremelanotide for the treatment of hypoactive sexual desire disorder in premenopausal women. CNS Spectr. 2022;27(3):281-289. https://doi.org/10.1017/S109285292100002X ↩︎
FDA. VYLEESI (bremelanotide injection) Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210557s000lbl.pdf ↩︎