¶ At a Glance
What is it?
Semaglutide is a long-acting analogue of the human hormone glucagon-like peptide-1 (GLP-1). It mimics the body's natural satiety signals to regulate appetite, control blood sugar, and reduce cardiovascular risk.
Primary Benefit
Significant weight loss (approx. 15%) and major reduction in cardiovascular events (heart attack, stroke).
Safety Profile
CAUTION
Generally safe but requires strict titration to manage gastrointestinal side effects. Boxed Warning for potential thyroid C-cell tumors (based on rodent data). Contraindicated in personal/family history of Medullary Thyroid Carcinoma (MTC) or MEN 2.
¶ Protocol
| Variable | Recommendation |
|---|---|
| Dosage | Titrate from 0.25 mg to 2.4 mg weekly |
| Frequency | Once Weekly |
| Route | Subcutaneous Injection |
| Half-Life | ~1 week (160–168 hours) |
Clinical Note: Strict titration is critical to avoid severe nausea. Standard schedule:
- Month 1: 0.25 mg
- Month 2: 0.5 mg
- Month 3: 1.0 mg
- Month 4: 1.7 mg
- Month 5+: 2.4 mg (Maintenance)
¶ Benefits (The "Why")
¶ 1. Profound Weight Loss
Semaglutide drives significant weight reduction by targeting appetite centers in the brain (hypothalamus) to increase satiety and reduce cravings. In the landmark STEP 1 trial, participants lost an average of 14.9% of body weight, compared to just 2.4% in the placebo group[1][2].
¶ 2. Cardiovascular Protection
Beyond weight loss, Semaglutide offers direct cardioprotective benefits. The SELECT trial demonstrated a 20% reduction in major adverse cardiovascular events (MACE), including heart attack and stroke, in non-diabetic adults with obesity[3]. These benefits appear independent of the magnitude of weight loss, suggesting anti-inflammatory and endothelial improvements.
¶ 3. Metabolic & Glycemic Control
Originally approved for Type 2 Diabetes, it powerfully lowers HbA1c and improves insulin sensitivity. It stimulates insulin release only when blood sugar is high (glucose-dependent), minimizing the risk of hypoglycemia compared to older drugs like sulfonylureas.
¶ 4. Kidney Health
Emerging data from the FLOW trial (2024) indicates powerful renoprotective effects. It reduced the risk of kidney failure and death by 24% in diabetic patients with chronic kidney disease, leading to the trial being stopped early for efficacy[4][5].
¶ Mechanism of Action
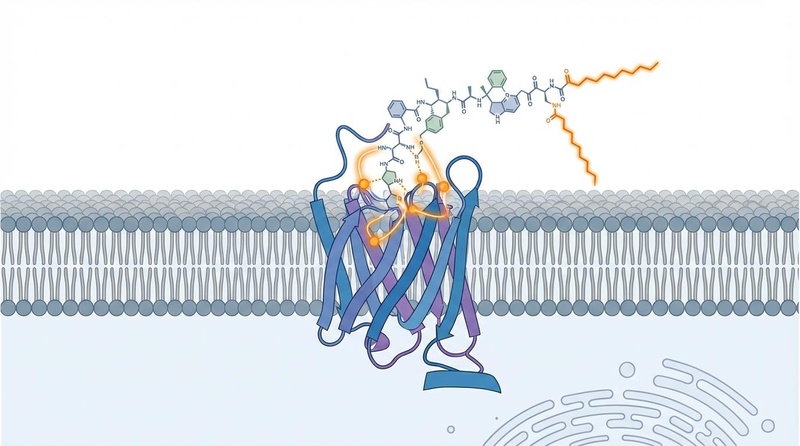
Semaglutide acts as a selective agonist for the GLP-1 receptor.
- Appetite Regulation: It crosses the blood-brain barrier to activate POMC/CART neurons (satiety) and inhibit NPY/AgRP neurons (hunger) in the hypothalamus. This reduces caloric intake and specifically diminishes cravings for high-fat/sugar foods.
- Gastric Emptying: It slows gastric emptying in the early post-dose phase, contributing to fullness and blunting post-meal glucose spikes.
- Pancreatic Function: It enhances glucose-dependent insulin secretion and suppresses inappropriate glucagon secretion.
- Systemic Effects: GLP-1 receptors in the heart and blood vessels mediate reductions in inflammation, blood pressure, and plasma lipids.
Molecular Engineering:
Native GLP-1 has a half-life of minutes. Semaglutide is modified (Aib substitution at position 8, C-18 fatty acid acylation) to resist degradation by DPP-4 and bind to albumin, extending its half-life to ~1 week.
¶ Evidence & Science
¶ Human Effect Matrix
| Outcome | Magnitude | Evidence Quality | Consistency |
|---|---|---|---|
| Weight Loss | High (~15%) | High (GRADE A) | High |
| HbA1c Reduction | High | High (GRADE A) | High |
| CV Risk Reduction | Moderate (20-26%) | High (GRADE A) | High |
| Kidney Protection | Moderate (24%) | High (GRADE A) | High |
| Alcohol Cravings | Moderate | Low (Emerging) | Moderate |
¶ Key Clinical Trials
- STEP 1 (Obesity): A randomized, double-blind trial (n=1,961) showing 14.9% mean weight loss with 2.4 mg Semaglutide vs. 2.4% with placebo. Over 86% of participants lost at least 5% of their body weight[1:1].
- SELECT (Cardiovascular): A massive trial (n=17,604) in non-diabetic patients with CVD and obesity. It proved that Semaglutide reduces the risk of CV death, non-fatal MI, or non-fatal stroke by 20%, establishing it as a cardiovascular drug, not just a weight loss drug[3:1].
- FLOW (Renal): Focused on Type 2 Diabetes with CKD (n=3,533). Semaglutide reduced the composite risk of kidney failure and mortality by 24%, demonstrating organ-protective effects distinct from glycemic control[4:1].
- SUSTAIN-6 (Diabetes CVOT): The initial safety trial (n=3,297) that showed a 26% reduction in MACE for diabetic patients, largely driven by a significant decrease in non-fatal stroke[6].
¶ Safety & Side Effects
¶ Common Side Effects
- Gastrointestinal: Nausea (>40%), vomiting, diarrhea, and constipation are very common, especially during dose escalation.
- Fatigue: Transient fatigue is often reported during the first weeks of treatment.
¶ Serious Risks
- Pancreatitis: Acute pancreatitis is a rare but serious risk. Monitor for severe abdominal pain radiating to the back.
- Gallbladder Disease: Rapid weight loss increases the risk of gallstones (cholelithiasis).
- Gastroparesis/Ileus: Rare post-marketing reports of severe stomach paralysis or bowel obstruction[7].
- Thyroid C-Cell Tumors: Boxed Warning. Contraindicated in patients with a history of Medullary Thyroid Carcinoma (MTC) or MEN 2 syndrome.
¶ Clinical Controversies
- Muscle Loss: Like all rapid weight loss, Semaglutide causes loss of both fat and lean mass. However, the lean-to-fat ratio improves, meaning fat is lost at a much higher rate than muscle. Resistance training and high protein intake are essential to mitigate sarcopenia[8].
- Ozempic Face: A colloquial term for facial volume loss due to rapid fat reduction.
¶ Legal Status
- FDA: Approved as Ozempic (T2D, 2017), Rybelsus (Oral T2D, 2019), and Wegovy (Obesity, 2021; CV indication, 2024).
- Shortage Status: As of Feb 2025, the FDA declared the shortage resolved, triggering a crackdown on compounding pharmacies producing unapproved copies[9].
- WADA: Not prohibited (as of 2026 Prohibited List). However, it is on the Monitoring Program (2025/2026) to track potential misuse for performance enhancement in weight-category sports[10].
¶ References
Wilding, J. P. H., et al. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine. https://pubmed.ncbi.nlm.nih.gov/33567185/ ↩︎ ↩︎
PubMed. (2021). STEP 1 Trial NEJM. https://pubmed.ncbi.nlm.nih.gov/33567185/ ↩︎
Lincoff, A. M., et al. (2023). Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. New England Journal of Medicine. https://pubmed.ncbi.nlm.nih.gov/37952131/ ↩︎ ↩︎
Perkovic, V., et al. (2024). Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. New England Journal of Medicine. https://pubmed.ncbi.nlm.nih.gov/38785209/ ↩︎ ↩︎
PubMed. (2024). FLOW Trial NEJM. https://pubmed.ncbi.nlm.nih.gov/38785209/ ↩︎
Marso, S. P., et al. (2016). Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa1607141 ↩︎
Healthline. (2023). FDA Updates Ozempic Label to Include Blocked Intestines. https://www.healthline.com/health-news/fda-updates-ozempic-label-to-include-blocked-intestines-as-potential-side-effect ↩︎
McCrimmon, R. J., et al. (2021). Impact of Semaglutide on Body Composition. PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC8089287/ ↩︎
Drug Topics. (2025). FDA Declares Shortage of Ozempic/Wegovy Resolved. https://www.drugtopics.com/view/fda-declares-shortage-of-ozempic-wegovy-resolved ↩︎
SwimSwam. (2025). Ozempic on WADA Monitoring List. https://swimswam.com/ozempic-on-wadas-monitoring-list-for-2025/ ↩︎