¶ Tirzepatide
Description: A "twincretin" dual GLP-1/GIP receptor agonist that delivers bariatric-surgery-level weight loss (~22.5%) and significant cardiovascular protection.
Tags: peptide, weight-loss, metabolic-health, diabetes, glp-1, gip, cardiovascular-health
¶ At a Glance
What is it?
Tirzepatide (brand names Mounjaro, Zepbound) is a "first-in-class" dual agonist peptide that activates both the GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. Unlike semaglutide (Ozempic/Wegovy) which targets only GLP-1, tirzepatide mimics the action of two natural hormones to produce synergistic effects on satiety, insulin secretion, and fat metabolism.
Primary Benefit
Unprecedented weight loss (up to 22.5% of body weight in 72 weeks) and profound improvements in glycemic control, lipid profiles, and cardiovascular risk factors.
Why use it?
It is currently the most potent pharmacological intervention for weight loss and Type 2 diabetes management available, offering results that approach bariatric surgery. Beyond weight loss, emerging data suggests significant potential for neuroprotection (reduced dementia risk) and cardiovascular health.
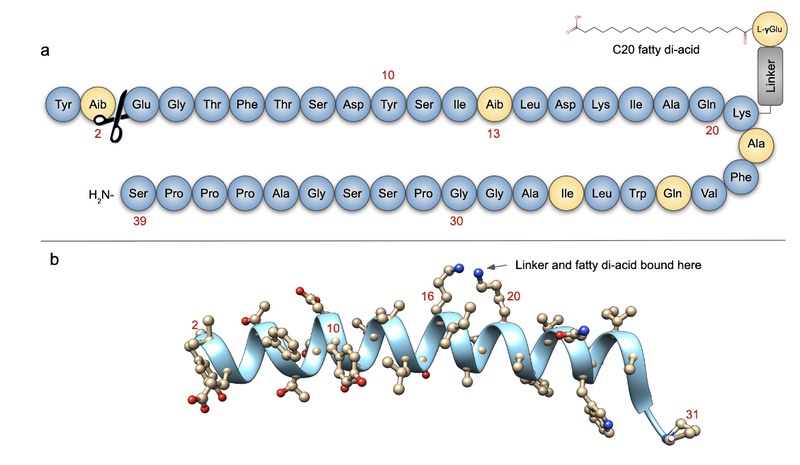
¶ Protocol
| Variable | Recommendation |
|---|---|
| Dosage | Start: 2.5 mg/week Max: 15 mg/week |
| Frequency | Once Weekly |
| Cycle | Continuous (Chronic management) |
| Route | Subcutaneous Injection (Abdomen, Thigh, Arm) |
¶ Standard Titration Schedule
Tirzepatide requires a slow titration to mitigate GI side effects. Do not rush the schedule.
- Month 1: 2.5 mg once weekly (Initiation dose, not therapeutic)
- Month 2: 5.0 mg once weekly
- Month 3: 7.5 mg once weekly
- Month 4: 10.0 mg once weekly
- Month 5: 12.5 mg once weekly
- Month 6+: 15.0 mg once weekly (Maximum maintenance dose)
Note: Many users stay at lower doses (5mg or 7.5mg) if they continue to see results and want to minimize side effects.
¶ Benefits (The "Why")
¶ 1. Unmatched Weight Loss
Tirzepatide has reset expectations for medical weight management. In the pivotal SURMOUNT-1 trial, non-diabetic adults lost an average of 15.0% (5 mg), 19.5% (10 mg), and 20.9% (15 mg) of their body weight over 72 weeks [1]. Over 57% of participants on the highest dose achieved >20% weight loss, a magnitude previously seen only with surgical interventions.
¶ 2. Cardiovascular & Heart Failure Protection
Beyond aesthetics, tirzepatide powerfully protects the heart.
- Heart Failure (HFpEF): The SUMMIT trial demonstrated a 38% reduction in the risk of cardiovascular death or worsening heart failure events (HR 0.62) in patients with obesity and heart failure with preserved ejection fraction [2].
- MACE Reduction: In type 2 diabetes, it proved non-inferior to dulaglutide for Major Adverse Cardiovascular Events (MACE), with trends favoring tirzepatide [3].
- Hypertension: Systolic blood pressure typically drops by 6–8 mmHg [1:1].
¶ 3. Metabolic & Glycemic Control
For type 2 diabetes, tirzepatide is superior to all comparators (including semaglutide 1 mg and insulin glargine) in reducing HbA1c (up to -2.30%) [4]. It also drastically lowers triglycerides (up to 27%) and improves insulin sensitivity, effectively reversing the metabolic dysfunction underlying type 2 diabetes.
¶ 4. Neuroprotection (Emerging)
There is growing excitement about the "neuro-metabolic" link. Insulin resistance in the brain is a key feature of Alzheimer's disease (sometimes called "Type 3 Diabetes").
- Risk Reduction: A large retrospective study (n>60,000) indicated that patients on GLP-1/GIP therapies like tirzepatide had a 37% lower risk of developing dementia compared to those on other diabetes drugs [5].
- Mechanism: Tirzepatide appears to reduce neuroinflammation and amyloid-β deposition while improving neuronal insulin signaling via the PI3K/AKT pathway.
¶ 5. Sleep Apnea Remission
The SURMOUNT-OSA trial showed that tirzepatide significantly reduced the Apnea-Hypopnea Index (AHI) by approximately 30 events per hour, leading to improved sleep quality and oxygenation in patients with obstructive sleep apnea [6].
¶ Mechanism of Action
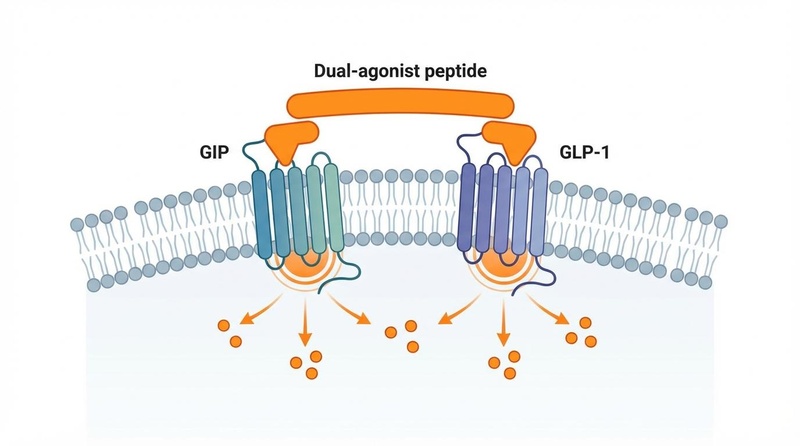
Tirzepatide's "twincretin" design targets two distinct pathways:
- GLP-1 Receptor Agonism (Weaker affinity): Like semaglutide, it acts on the hypothalamus to reduce appetite and "food noise," slows gastric emptying to prolong fullness, and enhances glucose-dependent insulin secretion.
- GIP Receptor Agonism (High affinity): Historically thought to be "diabetogenic," GIP agonism in this context appears to potentiate the weight-loss effects of GLP-1 while improving tolerability. It may directly target fat metabolism and insulin sensitivity in adipose tissue.
Biased Agonism: Tirzepatide binds to the GLP-1 receptor with lower potency (~18-fold weaker) than native GLP-1 but activates the GIP receptor with full potency. This "imbalance" is hypothesized to be the key to its superior efficacy/tolerability ratio, allowing for more aggressive stimulation of metabolic pathways without triggering intolerable nausea [7].
¶ Evidence & Science
¶ Human Effect Matrix
| Outcome | Magnitude | Evidence Quality | Consistency |
|---|---|---|---|
| Weight Loss (Non-Diabetic) | High (↓22.5%) | High (SURMOUNT-1) | High |
| Weight Loss (T2 Diabetes) | High (↓15.7%) | High (SURMOUNT-2) | High |
| HbA1c Reduction | High (↓2.3%) | High (SURPASS) | High |
| HFpEF Events | Mod (HR 0.62) | High (SUMMIT) | High |
| Sleep Apnea (AHI) | High (↓30/hr) | High (SURMOUNT-OSA) | High |
| MACE (CV Events) | Neutral/Pos | High (SURPASS-CVOT) | High |
¶ Key Studies
- SURMOUNT-1 (2022): The landmark Phase 3 trial in 2,539 adults with obesity. Established the 15 mg dose as capable of inducing >20% weight loss, showing superiority over placebo with a similar safety profile to GLP-1 mono-agonists [1:2].
- SUMMIT (2024): A groundbreaking trial for Heart Failure with Preserved Ejection Fraction (HFpEF). Tirzepatide reduced the risk of worsening heart failure events or CV death by 38% (HR 0.62; p=0.026) and worsening HF events alone by 46% (HR 0.54) [2:1].
- SURPASS-2 (2021): Head-to-head comparison with semaglutide (1 mg). Tirzepatide (all doses) was superior to semaglutide in both HbA1c reduction and weight loss [4:1].
¶ Comparisons
¶ Tirzepatide vs. Semaglutide (Ozempic/Wegovy)
| Feature | Semaglutide | Tirzepatide |
|---|---|---|
| Mechanism | GLP-1 Agonist | Dual GIP/GLP-1 Agonist |
| Weight Loss (Max) | ~15-17% | ~20-25% |
| A1c Reduction | High | Very High (Superior in SURPASS-2) |
| Side Effects | Nausea common | Nausea common (potentially slightly higher) |
| Cost/Availability | High / Shortages | High / Shortages |
Verdict: Tirzepatide is the more potent agent. However, semaglutide has a longer track record of safety data (especially cardiovascular outcomes) which tirzepatide is still accumulating.
¶ Safety & Side Effects
- Thyroid Warning: Boxed warning for Thyroid C-cell tumors. Do NOT use if you have a personal or family history of Medullary Thyroid Carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Gastrointestinal Distress: Nausea, vomiting, and diarrhea are common, especially during dose escalation.
- Pancreatitis: Use with caution if you have a history of pancreatitis.
- Muscle Loss: Rapid weight loss can lead to lean muscle loss alongside fat; resistance training and adequate protein intake are critical.
Common:
- Nausea: Very common (>20%), especially during initiation and dose escalation.
- Gastrointestinal Distress: Diarrhea, constipation, vomiting, abdominal pain.
- Fatigue: Often linked to reduced caloric intake.
- Injection Site Reactions: Mild redness or itching.
Rare/Serious:
- Pancreatitis: Acute inflammation of the pancreas; requires immediate cessation.
- Gallbladder Disease: Gallstones (cholelithiasis) due to rapid weight loss.
- Hypoglycemia: Rare in non-diabetics; risk increases if used with insulin/sulfonylureas.
- Kidney Injury: Secondary to dehydration from severe GI side effects.
Contraindications:
- Thyroid C-Cell Tumors: Personal or family history of Medullary Thyroid Carcinoma (MTC) or Multiple Endocrine Neoplasia type 2 (MEN 2). (Based on rodent carcinogenicity data).
- Pregnancy/Breastfeeding: Discontinue at least 2 months before planned conception.
¶ Legal Status
- FDA: Approved. Marketed as Mounjaro for Type 2 Diabetes and Zepbound for Chronic Weight Management.
- WADA: Prohibited. Listed under S4 (Hormone and Metabolic Modulators). Athletes are banned from using tirzepatide at all times (in and out of competition).
¶ References
Jastreboff, A. M., et al. (2022). Tirzepatide Once Weekly for the Treatment of Obesity. New England Journal of Medicine, 387(3), 205–216. https://www.nejm.org/doi/full/10.1056/NEJMoa2206038 ↩︎ ↩︎ ↩︎
Eli Lilly. (2024). Lilly's tirzepatide successful in phase 3 study showing benefit in adults with heart failure with preserved ejection fraction and obesity (SUMMIT). [Press Release]. https://investor.lilly.com/news-releases/news-release-details/lillys-tirzepatide-successful-phase-3-study-showing-benefit ↩︎ ↩︎
Nicholls, S. J., et al. (2024). Cardiovascular Outcomes with Tirzepatide versus Dulaglutide in Type 2 Diabetes (SURPASS-CVOT). New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa2405928 ↩︎
Frías, J. P., et al. (2021). Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine, 385, 503-515. https://www.nejm.org/doi/full/10.1056/NEJMoa2107519 ↩︎ ↩︎
Wang, L., et al. (2024). Glucagon-like peptide-1 receptor agonists and risk of dementia in patients with type 2 diabetes: A population-based cohort study. eClinicalMedicine, 69. ↩︎
Malhotra, A., et al. (2024). Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa2404881 ↩︎
Willard, F. S., et al. (2020). Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight, 5(17), e140532. https://doi.org/10.1172/jci.insight.140532 ↩︎