¶ Hormesis
Hormesis is a biological phenomenon where exposure to low doses of a stressor or toxin induces a beneficial effect, whereas high doses of the same agent cause harm. This biphasic dose-response relationship is fundamental to understanding how lifestyle interventions like exercise, fasting, and sauna use promote health and longevity. By triggering adaptive cellular stress response pathways, hormesis enhances an organism's resilience, maintenance, and repair mechanisms [1][2].
¶ Definition and The Dose-Response Curve
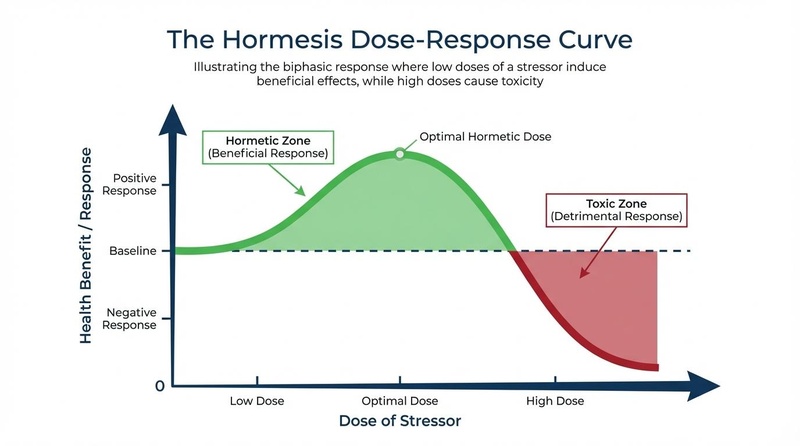
The core concept of hormesis is that the dose makes the poison—and the cure. Unlike linear models of toxicity, where any amount of a stressor is considered harmful, the hormetic model describes a J-shaped or inverted U-shaped curve:
- Low Dose (Eustress): Mild stress disrupts cellular homeostasis slightly, triggering an overcompensation in repair and defense mechanisms. This results in a net physiological benefit.
- High Dose (Distress): Severe stress overwhelms the body's adaptive capacity, leading to damage, toxicity, or death.
¶ The "Goldilocks Zone"
For hormesis to be effective, the intensity and duration of the stress must fall within a specific "Goldilocks zone"—not too little to be ineffective, and not too much to be damaging. This optimal zone represents the sweet spot where the adaptive response maximizes healthspan and resilience [3]. For example, while moderate exercise builds muscle and mitochondrial capacity, extreme overtraining can lead to injury and immune suppression.
¶ Mechanisms of Action
Hormetic stressors do not directly improve health; rather, they signal the body to upgrade its own defenses. Key molecular pathways involved include:
¶ NRF2 (Antioxidant Defense)
Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) is the "master regulator" of the antioxidant response. Hormetic triggers (like oxidative stress from exercise or sulforaphane) cause NRF2 to translocate to the nucleus, where it upregulates cytoprotective genes, including superoxide dismutase (SOD) and glutathione S-transferase [4][5]. This protects cells from chronic oxidative damage and inflammation [6].
¶ Heat Shock Proteins (Proteostasis)
Heat Shock Proteins (HSPs), such as HSP70, are molecular chaperones induced by thermal stress (heat/cold) and other insults. They ensure proper protein folding, prevent aggregation of misfolded proteins, and facilitate the removal of damaged proteins [7]. Maintaining proteostasis via HSPs is critical for preventing age-related diseases like Alzheimer's [8].
¶ AMPK (Energy Sensing)
AMP-Activated Protein Kinase (AMPK) detects low energy states (high AMP:ATP ratio), such as during fasting or intense exercise. Activation of AMPK inhibits anabolic pathways (like mTOR) and stimulates catabolic processes, including fatty acid oxidation and mitochondrial biogenesis [9]. It is a central regulator of metabolic health and longevity [10].
¶ Sirtuins (Epigenetic Regulation)
Sirtuins (SIRT1–SIRT7) are NAD+-dependent deacetylases that link metabolism to longevity. Activated by high NAD+ levels (common in energy-depleted states), sirtuins repair DNA, maintain genomic stability, and regulate inflammation [11][12].
¶ Autophagy (Cellular Recycling)
Autophagy is the cellular quality control process that degrades and recycles damaged organelles (like mitochondria) and proteins. Hormetic stressors like fasting and rapamycin potently stimulate autophagy, preventing the accumulation of cellular "garbage" that drives aging [13].
¶ Mitohormesis
Mitohormesis challenges the traditional view that mitochondrial Reactive Oxygen Species (ROS) are purely harmful. It proposes that low levels of ROS produced during exercise or caloric restriction act as essential signaling molecules. These signals trigger adaptive responses (such as increased antioxidant production) that ultimately extend lifespan [14][15]. Blocking these ROS signals with high-dose antioxidants can blunt the benefits of exercise [16][17].
¶ Types of Hormetic Stressors
¶ Physical: Exercise
Exercise is the quintessential hormetic stressor, causing temporary muscle damage, oxidative stress, and energy depletion.
- Mechanisms: Activates AMPK, mTOR (in recovery), and mitochondrial biogenesis.
- Benefits: Improved cardiovascular health, muscle mass, insulin sensitivity, and cognitive function [18][19].
¶ Thermal: Heat and Cold
- Sauna (Heat Stress): Exposure to heat (80–100°C) mimics moderate exercise, increasing heart rate and releasing HSPs. Regular use is linked to reduced cardiovascular mortality [20][21].
- Cold Plunge (Cold Stress): Cold exposure triggers vasoconstriction, norepinephrine release, and brown fat activation. It enhances immune function and metabolic resilience [20:1][22].
¶ Nutritional: Fasting and Caloric Restriction
- Caloric Restriction (CR): Reducing calorie intake without malnutrition is a robust method to extend lifespan in model organisms [23].
- Intermittent Fasting (IF): Cycling between eating and fasting depletes glycogen, lowers insulin, and activates autophagy [24][25].
¶ Xenohormesis: Plant Compounds
Xenohormesis is the hypothesis that animals benefit from ingesting stress-signaling molecules produced by plants [26]. These phytochemicals are mild toxins that trigger our defense pathways:
- Sulforaphane: Found in broccoli sprouts; activates NRF2 [27].
- Resveratrol: Found in grapes; mimics CR and activates SIRT1 [28].
- Curcumin: Found in turmeric; modulates inflammation and NRF2 [29].
¶ Hypoxia
Intermittent Hypoxic Training (IHT) involves brief exposure to low-oxygen environments. This stabilizes HIF-1α, improving oxygen transport, erythropoietin (EPO) production, and mitochondrial efficiency [30][31].
¶ Relevance to Aging and Longevity
Aging is characterized by a progressive loss of resilience and the accumulation of damage. Hormesis directly counters these processes by:
- Enhancing Maintenance: Upregulating repair systems (DNA repair, autophagy) that naturally decline with age.
- Building Resilience: Creating a "biological reserve" that allows the organism to withstand future stressors (e.g., infections, surgery) [2:1].
- Extending Healthspan: Regular exposure to hormetic stressors is associated with lower risks of chronic diseases and extended functional longevity [32][33].
¶ Safety and Considerations
While hormesis is beneficial, the dose-response nature implies risks:
- Overtraining/Overstress: Exceeding the "Goldilocks zone" leads to maladaptation, injury, and immune suppression.
- Individual Variability: The optimal dose varies by age, health status, and genetics. What is hormetic for a young, healthy person may be toxic for a frail, elderly individual.
- Recovery: The beneficial adaptation occurs during the recovery phase, not the stress phase. Adequate sleep and nutrition are essential for hormesis to work.
¶ References
Calabrese EJ. Hormesis: a fundamental concept in biology. Microb Cell. 2015. https://search.proquest.com/openview/4e3442cac29beb628d0b6006383e6613/1?pq-origsite=gscholar&cbl=55378 ↩︎
Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. Ageing Res Rev. 2023. https://radiationeffects.org/wp-content/uploads/2023/09/Ageing-Res-Reviews-Hormesis-and-Longspan-1.pdf ↩︎ ↩︎
Le Bourg E. Hormesis, aging and longevity. Biochim Biophys Acta. 2025. https://www.researchgate.net/publication/26235147_Hormesis_aging_and_longevity ↩︎
Zhang DD. Hormesis and polyphenol mediate Nrf2 regulation of disease and health. ResearchGate. 2024. https://www.researchgate.net/figure/Hormesis-and-polyphenol-mediate-Nrf2-regulation-of-disease-and-health-Heat-shock_fig3_382240492 ↩︎
Role of HSPs and autophagy in mechanisms underlying effects of sulforaphane. Physiol Res. 2023. https://www.biomed.cas.cz/physiolres/pdf/72/72_S47.pdf ↩︎
Nrf2 in Alzheimer's disease and hormesis. PubMed. 2023. https://pubmed.ncbi.nlm.nih.gov/36656673/ ↩︎
Cell Stress Chaperones. Mechanisms of autophagy in the proteotoxic stress response. Cell Stress Chaperones. 2023. https://pmc.ncbi.nlm.nih.gov/articles/PMC10050656/ ↩︎
Hormetic Heat Shock Enhances Autophagy through HSF1. Cells. 2022. https://pubmed.ncbi.nlm.nih.gov/35681472/ ↩︎
AMPK and sirtuins in aging. PMC. 2024. https://pmc.ncbi.nlm.nih.gov/articles/PMC12539535/ ↩︎
Endurance exercise-induced muscle phenotypes and autophagy. PMC. 2024. https://pmc.ncbi.nlm.nih.gov/articles/PMC11298286/ ↩︎
Linking Sirtuins and AMPK in Metabolism and Aging. PMC. 2010. https://pmc.ncbi.nlm.nih.gov/articles/PMC2853213/ ↩︎
Mechanism by which AMPK and sirtuin proteins regulate metabolism. ResearchGate. 2023. https://www.researchgate.net/figure/Mechanism-by-which-AMPK-and-sirtuin-proteins-regulate-metabolism-and-resist-aging_fig2_374872000 ↩︎
Autophagy and hormesis. PMC. 2011. https://pmc.ncbi.nlm.nih.gov/articles/PMC3227447/ ↩︎
Ristow M. Unraveling the Truth About Antioxidants: Mitohormesis explains ROS-induced health benefits. Nat Med. 2014. https://pmc.ncbi.nlm.nih.gov/articles/PMC4036400/ ↩︎
Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response. 2014. https://www.semanticscholar.org/paper/Mitohormesis%3A-Promoting-Health-and-Lifespan-by-of-Ristow-Schmeisser/ad0f122bf8ad2248bbd2365321ec2bc121c6fd77 ↩︎
Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010. https://pubmed.ncbi.nlm.nih.gov/20350594/ ↩︎
Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response. 2014. https://scholarworks.umass.edu/items/287509c8-2810-4db5-873f-62e3a8346e92 ↩︎
Are there specific types of exercise that have a more significant impact on autophagy? Consensus. 2024. https://consensus.app/search/are-there-specific-types-of-exercise-that-have-a-m/WBgbNZdtSjqrdlEw5dFxKw/ ↩︎
Sanchez AM, et al. Exercise and autophagy in skeletal muscle. ResearchGate. 2014. https://www.researchgate.net/figure/Exercise-and-autophagy-in-skeletal-muscle-While-a-single-boot-of-endurance-exercise_fig3_264794740 ↩︎
The Science of Sauna and Cold Plunge. Nirvana Islands. 2025. https://www.nirvanaislands.com/insights/the-science-of-sauna-and-cold-plunge-evidence-based-health-benefits ↩︎ ↩︎
Sauna and Longevity. Lifespan.io. 2022. https://www.lifespan.io/topic/sauna-longevity/ ↩︎
Heat and Cold Protocols. Huberman Lab. 2024. https://ai.hubermanlab.com/s/987a185c-e0cf-11ee-9501-7b99e0e1dc0b ↩︎
Fasting: Molecular Mechanisms and Clinical Applications. PMC. 2014. https://pmc.ncbi.nlm.nih.gov/articles/PMC3946160/ ↩︎
Human studies on prolonged fasting/fasting-mimicking diet. PMC. 2022. https://pmc.ncbi.nlm.nih.gov/articles/PMC8932957/ ↩︎
Fasting and Hormesis. YouTube. 2022. https://www.youtube.com/watch?v=tolvxXh5RqE ↩︎
Hooper PL, et al. Xenohormesis: health benefits from an eon of plant stress response. J Evol Med. 2010. https://pmc.ncbi.nlm.nih.gov/articles/PMC3024065/ ↩︎
Sulforaphane vs Curcumin vs Resveratrol. VitaFenix. 2025. https://vitafenixsupplements.com/blog/sulforaphane-vs-curcumin-vs-resveratrol/ ↩︎
Xenohormesis: health benefits from an eon of plant stress response. PMC. 2016. https://pmc.ncbi.nlm.nih.gov/articles/PMC4775249/ ↩︎
Curcumin as a hormetic agent. PubMed. 2018. https://pubmed.ncbi.nlm.nih.gov/30515809/ ↩︎
Hormesis Determines Lifespan: Hypoxia. ResearchGate. 2025. https://www.researchgate.net/publication/377121416_HORMESIS_DETERMINES_LIFESPAN ↩︎
Safety and efficacy of intermittent hypoxia conditioning. PMC. 2020. https://pmc.ncbi.nlm.nih.gov/articles/PMC8950503/ ↩︎
Calabrese EJ, et al. Hormesis determines lifespan. Biogerontology. 2024. https://pubmed.ncbi.nlm.nih.gov/38182079/ ↩︎
Calabrese EJ, et al. Hormesis determines lifespan. Biogerontology. 2023. https://scispace.com/papers/hormesis-determines-lifespan-ux7f3gp774 ↩︎