¶ MK-677 (Ibutamoren): Benefits, Dosage, & Side Effects
| Sequence | Non-peptide (Small Molecule) |
| Formula | C27H36N4O5S (Mesylate: C28H40N4O8S2) |
| Molar Mass | 528.67 g/mol (Free base) |
| Category | GH Secretagogue (Ghrelin Agonist) |
| Half-life | ~24 hours |
| Admin | Oral (Capsule/Liquid) |
| FDA Status | Not Approved / Category 2 (Restricted) |
| CAS | 159752-10-0 (Mesylate) |
MK-677 (also known as Ibutamoren or L-163,191) is a potent, long-acting, orally-active growth hormone secretagogue (GHS). Unlike injectable peptides (like Ipamorelin or CJC-1295), MK-677 is a non-peptide small molecule that mimics the action of the hunger hormone ghrelin.
It is widely used in longevity and bodybuilding circles because it offers the benefits of elevated Growth Hormone (GH) and IGF-1—such as improved recovery and muscle preservation—without the need for injections. However, its "dirty" side effect profile (including extreme hunger, water retention, and insulin resistance) and recent FDA crackdowns make it a controversial compound.
¶ At a glance
Aliases
- Generic Name: Ibutamoren, Ibutamoren Mesylate
- Research Codes: MK-677, MK-0677, L-163,191, LUM-201
- Category: Ghrelin Receptor Agonist, Oral GHS
Key points
- Primary Benefit: Sustained, robust elevation of IGF-1 levels (often 40-90% increase) with a single daily oral dose.
- Notable Effect: Stimulates intense hunger ("ghrelin munchies") similar to GHRP-6, which can be useful for bulking but detrimental for diet adherence.
- Key Limitation: Significant risk of insulin resistance (diabetogenic effect) and water retention (edema), which can strain the cardiovascular system.
- Safety Warning: Clinical trials for Alzheimer's and frailty failed to show functional benefit and, in one hip-fracture study, showed an increased risk of congestive heart failure in elderly patients[1].
What people use it for
- Main goals: Muscle mass retention (anti-catabolic), injury recovery, bone density improvement, and sleep quality enhancement.
- Evidence quality: High for physiological effects (raising IGF-1/GH); Low/Mixed for clinical outcomes (failed in frailty/Alzheimer's trials).
¶ Legal & regulatory status
As of late 2023/2024, the FDA placed MK-677 on the Category 2 list of bulk drug substances (Safety Concerns). This classification effectively prohibits U.S. compounding pharmacies from producing MK-677. The FDA cited potential risks of congestive heart failure in elderly patients and the lack of approved medical use.
Regulatory classification
- FDA Status: Unapproved. MK-677 is an Investigational New Drug (IND) that was never approved for any medical indication.
- Dietary Supplements: It is illegal to sell MK-677 as a dietary supplement. The FDA frequently issues warning letters to companies marketing it as such.
Sports and competition
- WADA Status: Prohibited. Listed under S2. Peptide Hormones, Growth Factors, Related Substances, and Mimetics. Banned at all times (in and out of competition).
¶ What is MK-677?
MK-677 is a non-peptide spiropiperidine developed by Merck.
- Mechanism: It acts as a selective agonist of the ghrelin receptor (GHS-R1a) in the pituitary gland and hypothalamus.
- Ghrelin Mimicry: Like the peptide GHRP-6, it tricks the body into thinking ghrelin levels are high. This triggers:
- GH Release: The pituitary dumps growth hormone.
- Appetite: The hypothalamus signals intense hunger.
- Pharmacokinetics: Unlike peptides which are destroyed by digestion, MK-677 is stable orally and has a long half-life (~24 hours), maintaining elevated IGF-1 levels around the clock.
¶ What are MK-677's main benefits?
1. Muscle Preservation (Anti-Catabolic)
- Outcome: Increases Fat-Free Mass (FFM).
- Evidence: A study in healthy young men showed that MK-677 reversed diet-induced nitrogen wasting (muscle loss) during a caloric deficit[2]. In older adults, it increased FFM by ~1.6 kg over 2 years[3].
- Caveat: Much of this "lean mass" is likely intracellular water retention rather than contractile muscle tissue.
2. Bone Density
- Outcome: Increased markers of bone turnover and bone mineral density (BMD).
- Evidence: Trials in elderly subjects and obese men demonstrated increased bone remodeling and density, suggesting potential (though unproven) utility for osteoporosis.
3. Sleep Quality
- Outcome: Improvement in REM sleep duration and quality.
- Mechanism: Ghrelin signaling is linked to sleep-wake regulation. Users often report deeper sleep and vivid dreams.
4. Injury Recovery
- Mechanism: Systemic elevation of IGF-1 promotes collagen synthesis and tissue repair, similar to HGH therapy.
¶ Evidence summary table (human outcomes)
| Outcome / Goal | Effect* | Consistency** | Evidence quality | Trials | Notes |
|---|---|---|---|---|---|
| Increase IGF-1 | High | High | Multiple | Consistently raises IGF-1 by 40-90% within weeks; sustained for >1 year[3:1]. | |
| Muscle Mass (FFM) | High | High | 3+ RCTs | Increases lean mass, but partly due to water; did not improve strength/function in frail elderly[3:2][1:1]. | |
| Fat Loss | ↔ / ↓ | Low | Moderate | Mixed | GH burns fat, but the hunger effect often leads to increased caloric intake, neutralizing fat loss. |
| Cognitive Function | High | High | 1 Large RCT | Failed to slow progression of Alzheimer's Disease in a major Phase IIb trial[4]. | |
| Insulin Sensitivity | High | High | Multiple | Consistently increases fasting glucose and reduces insulin sensitivity (diabetogenic)[3:3]. |
¶ How does MK-677 work?
The Ghrelin Pathway
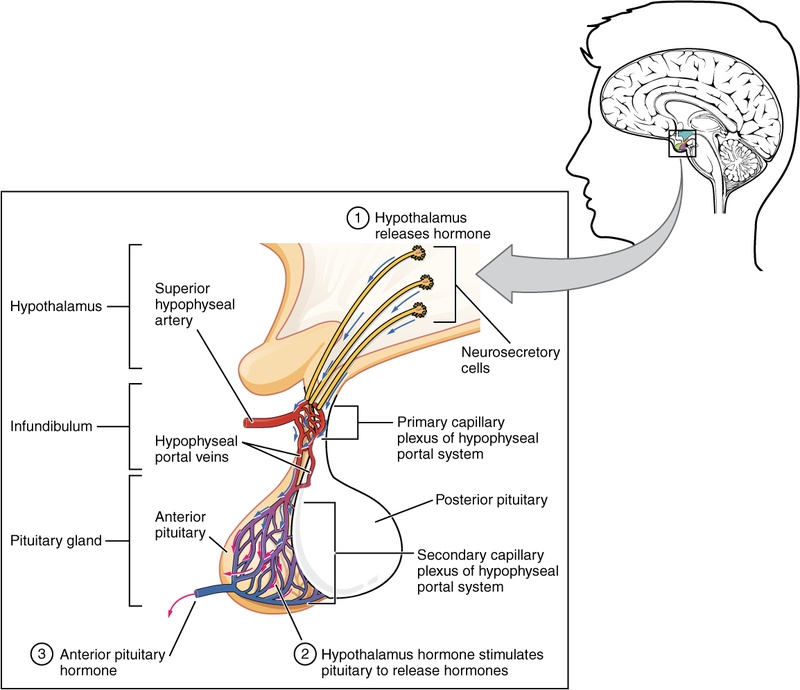
- Receptor Activation: MK-677 binds to GHS-R1a receptors.
- GH Pulse Amplification: It amplifies the natural pulsatile release of GH.
- IGF-1 Conversion: The liver converts the released GH into Insulin-Like Growth Factor 1 (IGF-1), which mediates most anabolic effects.
- Cortisol Sparing: Unlike early generation secretagogues, MK-677 generally increases GH without causing a chronic elevation in cortisol, though transient rises can occur.
¶ Administration and Dosing
Standard Dosage
- Range: 10 mg to 25 mg per day.
- Plateau: Clinical data suggests that doses above 25 mg do not significantly increase IGF-1 further, but do increase side effects (insulin resistance, edema)[5].
- Form: Oral capsule or liquid solution.
Timing
- Morning: Taken with breakfast. Recommended to blunt the hunger spike (since you are eating anyway).
- Night: Taken before bed. May improve sleep, but some users report waking up starving.
Cycling
- Duration: Typically 8–16 weeks.
- "5 On / 2 Off": A common protocol (Mon-Fri on, weekends off) attempted to mitigate insulin desensitization, though the long half-life makes the efficacy of this strategy debatable.
¶ Safety and side effects
MK-677 has a "dirtier" safety profile than selective peptides like Ipamorelin.
1. Insulin Resistance (Diabetogenic)
- Risk: High. GH counteracts insulin. MK-677 consistently raises fasting blood glucose and HbA1c.
- Management: Users often pair it with Berberine (500mg 3x/day) or Metformin to manage blood sugar. It is contraindicated for diabetics.
2. Fluid Retention (Edema)
- Symptoms: Swollen ankles, tight rings, "moon face."
- Risk: Excess fluid increases blood pressure and cardiac strain.
- FDA Warning: The hip-fracture study was terminated early due to a higher rate of congestive heart failure in the MK-677 group (related to fluid overload in frail patients)[1:2].
3. Extreme Hunger
- Mechanism: Ghrelin agonism.
- Impact: Can be unbearable for some, leading to binge eating. Useful for "hard gainers" (bulking) but bad for cutting.
4. Prolactin Elevation
- Risk: Mild to Moderate. Can cause nipple sensitivity or gynecomastia in sensitive individuals.
¶ Drug interactions
- Insulin / Secretagogues: May require dose adjustment due to MK-677's hyperglycemic effect.
- Glucocorticoids: May oppose the anabolic effects.
¶ Practical questions (FAQ)
1. Is MK-677 a SARM?
No. It is often sold alongside SARMs (Selective Androgen Receptor Modulators) but it is a Secretagogue. It does not affect testosterone or bind to androgen receptors, so it does not require PCT (Post Cycle Therapy) for testosterone recovery.
2. Will it make me fail a drug test?
Yes, if it is a WADA/sports test. Standard employment drug tests (5-panel, 10-panel) generally do not look for it.
3. Can I stack it with peptides?
Yes. It is often stacked with CJC-1295 (to amplify the GH pulse signal). However, stacking two secretagogues increases the risk of side effects like insulin resistance.
4. Why was it banned for compounding?
The FDA cited the heart failure signal in the hip-fracture trial and the lack of any approved medical use as reasons to classify it as a safety risk (Category 2).
¶ References
Adunsky A, et al. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr. 2011;53(2):183-9. PubMed ↩︎ ↩︎ ↩︎
Murphy MG, et al. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol Metab. 1998;83(2):320-5. PubMed ↩︎
Nass R, et al. Effects of an oral growth hormone secretagogue (MK-677) on physical function and body composition in healthy older adults: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2008;149(9):601-11. PubMed ↩︎ ↩︎ ↩︎ ↩︎
Sevigny JJ, et al. Growth hormone secretagogue MK-677: No clinical effect on AD progression in a randomized trial. Neurology. 2008;71(20):1634-8. PubMed ↩︎
Copinschi G, et al. Effects of a 7-day treatment with a novel, orally active, growth hormone (GH) secretagogue, MK-677, on 24-hour GH profiles. J Clin Endocrinol Metab. 1996;81(7):2376-81. PubMed ↩︎